Alpha-1 Antitrypsin-Induced Endoplasmic Reticulum Stress Promotes Invasion by Extravillous Trophoblasts
- PMID: 33916165
- PMCID: PMC8037753
- DOI: 10.3390/ijms22073683
Alpha-1 Antitrypsin-Induced Endoplasmic Reticulum Stress Promotes Invasion by Extravillous Trophoblasts
Abstract
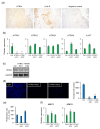
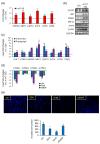
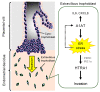
Alpha-1 antitrypsin (A1AT) is a glycoprotein that has been shown to protect tissues from proteolytic damage under various inflammatory conditions. Several studies show that A1AT may be associated with pre-eclampsia. However, the role of A1AT expression in placental physiology is not fully understood. In the present study, we aim to characterize the expression and function of placental A1AT. A1AT knockdown is found to reduce the expression of the serine protease HTRA1 in a trophoblast cell line. In addition, A1AT overexpression (A1AT-OE) increases the expression of HTRA1, IL6, CXCL8, and several markers of endoplasmic reticulum (ER) stress. Treatment with tunicamycin or thapsigargin, which induces ER stress, increases HTRA1 expression. Furthermore, immunohistochemistry reveals that HTRA1 is expressed in trophoblasts and the endometrial decidual cells of human placentas. An invasion assay shows that A1AT and HTRA1 stimulate cell invasion, but treatment with the ER stress inhibitors reduces the expression of HTRA1 and ER stress markers and prevents cell invasion in A1AT-OE trophoblasts. These results suggest that endogenous A1AT regulates inflammatory cytokine expression and HTRA1-induced trophoblast invasion via the induction of ER stress. It is concluded that an imbalance in the functional link between A1AT and ER stress at the maternal-fetal interface might cause abnormal placental development.
Keywords: alpha-1 antitrypsin; cell invasion; endoplasmic reticulum stress; extravillous trophoblast; high-temperature requirement A serine peptidase 1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Alpha 1 Antitrypsin Regulates Trophoblast Syncytialization and Inflammatory Factor Expression.Int J Mol Sci. 2022 Feb 10;23(4):1955. doi: 10.3390/ijms23041955. Int J Mol Sci. 2022. PMID: 35216073 Free PMC article.
-
Endoplasmic reticulum stress-regulated high temperature requirement A1 (HTRA1) modulates invasion and angiogenesis-related genes in human trophoblasts.J Pharmacol Sci. 2022 Dec;150(4):267-274. doi: 10.1016/j.jphs.2022.10.003. Epub 2022 Oct 7. J Pharmacol Sci. 2022. PMID: 36344049
-
Placental HTRA1 cleaves α1-antitrypsin to generate a NET-inhibitory peptide.Blood. 2021 Sep 16;138(11):977-988. doi: 10.1182/blood.2020009021. Blood. 2021. PMID: 34192300 Free PMC article.
-
The interaction of ER stress and autophagy in trophoblasts: navigating pregnancy outcome†.Biol Reprod. 2024 Aug 15;111(2):292-311. doi: 10.1093/biolre/ioae066. Biol Reprod. 2024. PMID: 38678504 Review.
-
Well-Known and Less Well-Known Functions of Alpha-1 Antitrypsin. Its Role in Chronic Obstructive Pulmonary Disease and Other Disease Developments.Ann Am Thorac Soc. 2016 Aug;13 Suppl 4:S280-8. doi: 10.1513/AnnalsATS.201507-468KV. Ann Am Thorac Soc. 2016. PMID: 27564662 Review.
Cited by
-
Protein Misfolding in Pregnancy: Current Insights, Potential Mechanisms, and Implications for the Pathogenesis of Preeclampsia.Molecules. 2024 Jan 26;29(3):610. doi: 10.3390/molecules29030610. Molecules. 2024. PMID: 38338354 Free PMC article. Review.
-
Alpha 1 Antitrypsin Regulates Trophoblast Syncytialization and Inflammatory Factor Expression.Int J Mol Sci. 2022 Feb 10;23(4):1955. doi: 10.3390/ijms23041955. Int J Mol Sci. 2022. PMID: 35216073 Free PMC article.
-
Role and mechanism of alpa1-antitrypsin in polycystic ovary syndrome.Saudi Med J. 2022 Dec;43(12):1309-1316. doi: 10.15537/smj.2022.43.12.20210398. Saudi Med J. 2022. PMID: 36517050 Free PMC article.
-
Injectable Peptide Hydrogel Encapsulation of Mesenchymal Stem Cells Improved Viability, Stemness, Anti-Inflammatory Effects, and Early Stage Wound Healing.Biomolecules. 2022 Sep 17;12(9):1317. doi: 10.3390/biom12091317. Biomolecules. 2022. PMID: 36139156 Free PMC article.
-
Insufficient GDF15 expression predisposes women to unexplained recurrent pregnancy loss by impairing extravillous trophoblast invasion.Cell Prolif. 2023 Dec;56(12):e13514. doi: 10.1111/cpr.13514. Epub 2023 Jun 4. Cell Prolif. 2023. PMID: 37272232 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

