Removing of the Sulfur Compounds by Impregnated Polypropylene Fibers with Silver Nanoparticles-Cellulose Derivatives for Air Odor Correction
- PMID: 33916200
- PMCID: PMC8067035
- DOI: 10.3390/membranes11040256
Removing of the Sulfur Compounds by Impregnated Polypropylene Fibers with Silver Nanoparticles-Cellulose Derivatives for Air Odor Correction
Abstract
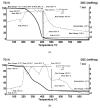
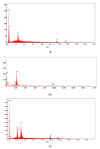
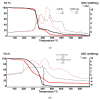
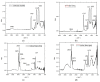
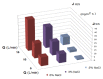
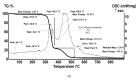
The unpleasant odor that appears in the industrial and adjacent waste processing areas is a permanent concern for the protection of the environment and, especially, for the quality of life. Among the many variants for removing substance traces, which give an unpleasant smell to the air, membrane-based methods or techniques are viable options. Their advantages consist of installation simplicity and scaling possibility, selectivity; moreover, the flows of odorous substances are direct, automation is complete by accessible operating parameters (pH, temperature, ionic strength), and the operation costs are low. The paper presents the process of obtaining membranes from cellulosic derivatives containing silver nanoparticles, using accessible raw materials (namely motion picture films from abandoned archives). The technique used for membrane preparation was the immersion precipitation for phase inversion of cellulosic polymer solutions in methylene chloride: methanol, 2:1 volume. The membranes obtained were morphologically and structurally characterized by scanning electron microscopy (SEM) and high resolution SEM (HR SEM), energy dispersive X-ray analysis (EDAX), Fourier transform infrared spectrometry (FTIR), thermal analysis (TG, ATD). Then, the membrane performance process (extraction efficiency and species flux) was determined using hydrogen sulfide (H2S) and ethanethiol (C2H5SH) as target substances.
Keywords: air odor correction; cellulose; ethanethiol; hydrogen sulfide; membrane processes; membranes; polypropylene fibers; silver nanoparticles; sulfur compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures















References
-
- Beck J.F., Cormier F., Donini J.C. The combined toxicity of ethanol and hydrogen sulfide. Toxicol. Lett. 1979;3:311–313. doi: 10.1016/0378-4274(79)90009-2. - DOI
-
- Layton D.W., Cederwall R.T. Assessing and managing the risks of accidental releases of hazardous gas: A case study of natural gas wells contaminated with hydrogen sulfide. Environ. Int. 1986;12:519–532. doi: 10.1016/0160-4120(86)90146-7. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

