ANCA-Associated Vasculitis: An Update
- PMID: 33916214
- PMCID: PMC8037363
- DOI: 10.3390/jcm10071446
ANCA-Associated Vasculitis: An Update
Abstract
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) represents a group of small vessel vasculitides characterized by granulomatous and neutrophilic tissue inflammation, often associated with the production of antibodies that target neutrophil antigens. The two major antigens targeted by ANCAs are leukocyte proteinase 3 (PR3) and myeloperoxidase (MPO). AAV can be classified into 3 categories based on patterns of clinical involvement: namely, granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA) and eosinophilic GPA (EGPA). Clinically, AAV involves many organ systems including the lungs, kidneys, skin, and nervous system. The prognosis of AAV has improved dramatically due to advances in the understanding of its pathogenesis and treatment modalities. This review will highlight some of the recent updates in our understanding of the pathogenesis, clinical manifestations, and treatment options in patients with AAV focusing on kidney involvement.
Keywords: ANCA vasculitis; crescentic glomerulonephritis; review; vasculitis.
Conflict of interest statement
Almaani reports receiving consulting fees from Aurinia, Kezar and grant support from Gilead paid to the Ohio State University. Jayne reports receiving consulting fees from AstraZeneca, ChemoCentryx, and Genentech and grant support, paid to the University of Cambridge, and consulting fees from GlaxoSmith-Kline. The rest of the authors declare no conflict of interest.
Figures

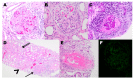
References
-
- Mohammad A.J., Jacobsson L.T., Mahr A.D., Sturfelt G., Segelmark M. Prevalence of Wegener’s granulomatosis, microscopic polyangiitis, polyarteritis nodosa and Churg-Strauss syndrome within a defined population in southern Sweden. Rheumatology. 2007;46:1329–1337. doi: 10.1093/rheumatology/kem107. - DOI - PubMed
-
- Knight A., Ekbom A., Brandt L., Askling J. Increasing incidence of Wegener’s granulomatosis in Sweden, 1975–2001. J. Rheumatol. 2006;33:2060–2063. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

