Synthesis of Fe Doped Poly p-Phenylenediamine Composite: Co-Adsorption Application on Toxic Metal Ions (F- and As3+) and Microbial Disinfection in Aqueous Solution
- PMID: 33916218
- PMCID: PMC8065817
- DOI: 10.3390/toxics9040074
Synthesis of Fe Doped Poly p-Phenylenediamine Composite: Co-Adsorption Application on Toxic Metal Ions (F- and As3+) and Microbial Disinfection in Aqueous Solution
Abstract
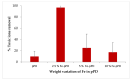
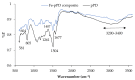
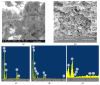
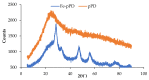
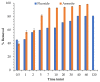
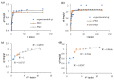
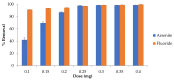
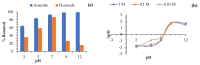
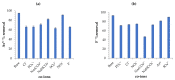
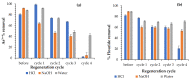
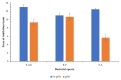
Water is regarded as an important natural resource to sustain life, and its purification is an important criterion that determines its quality and usefulness. In this study, the incorporation of Fe3+ oxide onto a phenylenediamine (pPD) polymer matrix through chemical co-polymerization was prepared, and its arsenite and fluoride removal potentials at optimal conditions from aqueous solution were evaluated. The morphology and structural analysis of the synthesized Fe-doped pPD (Fe-pPD) were comparatively evaluated using the FT-IR, SEM, EDS, and XRD techniques. Fe was successfully incorporated onto pPD matrix as confirmed by different morphological characterizations. The rate of adsorption of F- and As3+ onto the Fe-pPD composite best followed the pseudo-second-order kinetic model. The experimental data for both As3+ and F- onto the Fe-pPD composite better fit the Freundlich isotherm model at different operating temperatures. Overall, the synthesized composite exhibited a strong affinity towards fluoride uptake (96.6%) than arsenite uptake (71.14%) with a maximum capacity of 6.79 (F-) and 1.86 (As3+) mg/g. Additionally, the synthesized adsorbent showed some level of antimicrobial activity against common water-borne bacterial. Therefore, the Fe-doped pPD composite has the potential ability for inorganic metal species pollutants remediation and bacterial disinfection in community-level water purification processes.
Keywords: adsorption experiments; arsenic and fluoride remediation; bacterial disinfection; poly para-phenylenediamine composite; water pollution.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in the paper.
Figures

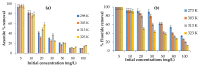

References
-
- WHO/UNICEF JMP (Joint Monitoring Programme for Water Supply and Sanitation) JMP Rapid Assessment on Drinking-Water Quality. Pilot Report. World Health Organization; Geneva, Switzerland: 2010. pp. 8–10.
-
- WHO/UNICEF . Progress on Drinking Water and Sanitation. 2014 Update. World Health Organization; Geneva, Switzerland: 2014.
-
- Thompson R.I., Eisenstein D., Fan X., Rieke M., Kennicutt R.C. Evidence for az <8 origin of the source-subtracted near-infrared background. Astrophys. J. 2007;666:658.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

