Adoptive T Cell Therapy for Solid Tumors: Pathway to Personalized Standard of Care
- PMID: 33916369
- PMCID: PMC8067276
- DOI: 10.3390/cells10040808
Adoptive T Cell Therapy for Solid Tumors: Pathway to Personalized Standard of Care
Abstract
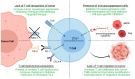
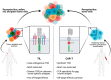
Adoptive cell therapy (ACT) with tumor-infiltrating T cells (TILs) has emerged as a promising therapy for the treatment of unresectable or metastatic solid tumors. One challenge to finding a universal anticancer treatment is the heterogeneity present between different tumors as a result of genetic instability associated with tumorigenesis. As the epitome of personalized medicine, TIL-ACT bypasses the issue of intertumoral heterogeneity by utilizing the patient's existing antitumor immune response. Despite being one of the few therapies capable of inducing durable, complete tumor regression, many patients fail to respond. Recent research has focused on increasing therapeutic efficacy by refining various aspects of the TIL protocol, which includes the isolation, ex vivo expansion, and subsequent infusion of tumor specific lymphocytes. This review will explore how the therapy has evolved with time by highlighting various resistance mechanisms to TIL therapy and the novel strategies to overcome them.
Keywords: adoptive cell therapy; immunotherapy; metastatic treatment; tumor-infiltrating T cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

