Inhibition of Cochlear HMGB1 Expression Attenuates Oxidative Stress and Inflammation in an Experimental Murine Model of Noise-Induced Hearing Loss
- PMID: 33916471
- PMCID: PMC8066810
- DOI: 10.3390/cells10040810
Inhibition of Cochlear HMGB1 Expression Attenuates Oxidative Stress and Inflammation in an Experimental Murine Model of Noise-Induced Hearing Loss
Abstract
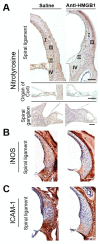
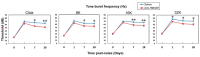
Noise-induced hearing loss (NIHL) is a common inner ear disease but has complex pathological mechanisms, one of which is increased oxidative stress in the cochlea. The high-mobility group box 1 (HMGB1) protein acts as an inflammatory mediator and shows different activities with redox modifications linked to the generation of reactive oxygen species (ROS). We aimed to investigate whether manipulation of cochlear HMGB1 during noise exposure could prevent noise-induced oxidative stress and hearing loss. Sixty CBA/CaJ mice were divided into two groups. An intraperitoneal injection of anti-HMGB1 antibodies was administered to the experimental group; the control group was injected with saline. Thirty minutes later, all mice were subjected to white noise exposure. Subsequent cochlear damage, including auditory threshold shifts, hair cell loss, expression of cochlear HMGB1, and free radical activity, was then evaluated. The levels of HMGB1 and 4-hydroxynonenal (4-HNE), as respective markers of reactive nitrogen species (RNS) and ROS formation, showed slight increases on post-exposure day 1 and achieved their highest levels on post-exposure day 4. After noise exposure, the antibody-treated mice showed markedly less ROS formation and lower expression of NADPH oxidase 4 (NOX4), nitrotyrosine, inducible nitric oxide synthase (iNOS), and intercellular adhesion molecule-1 (ICAM-1) than the saline-treated control mice. A significant amelioration was also observed in the threshold shifts of the auditory brainstem response and the loss of outer hair cells in the antibody-treated versus the saline-treated mice. Our results suggest that inhibition of HMGB1 by neutralization with anti-HMGB1 antibodies prior to noise exposure effectively attenuated oxidative stress and subsequent inflammation. This procedure could therefore have potential as a therapy for NIHL.
Keywords: NADPH oxidase (NOX); cochlea; high-mobility group box 1 (HMGB1); inflammation; noise-induced hearing loss (NIHL); oxidative stress; reactive nitrogen species (RNS); reactive oxygen species (ROS).
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
- MOST109-2314-B-016-005 to C.P.S. and MOST107-2314-B-663-001-MY3 to C.H.W./Ministry of Science and Technology, Taiwan.
- TSGH-A-109001 to C.P.S./Tri-Service General Hospital grants
- CAFGH-D-109025 to C.H.W., TCAFGH-E-109044 to H.C.C. and C.H.W./Taichung Armed Forces General Hospital grant
- A1071025 to H.C.C. and A1061019 to C.H.W./Teh-Tzer Study Group for Human Medical Research Foundation
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

