Antibacterial and Photocatalytic Properties of ZnO Nanoparticles Obtained from Chemical versus Saponaria officinalis Extract-Mediated Synthesis
- PMID: 33916520
- PMCID: PMC8038507
- DOI: 10.3390/molecules26072072
Antibacterial and Photocatalytic Properties of ZnO Nanoparticles Obtained from Chemical versus Saponaria officinalis Extract-Mediated Synthesis
Abstract
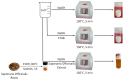
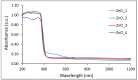
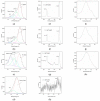
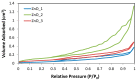
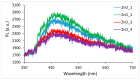
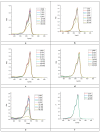
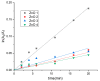
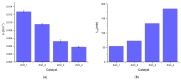
In the present work, the properties of ZnO nanoparticles obtained using an eco-friendly synthesis (biomediated methods in microwave irradiation) were studied. Saponaria officinalis extracts were used as both reducing and capping agents in the green nanochemistry synthesis of ZnO. Inorganic zinc oxide nanopowders were successfully prepared by a modified hydrothermal method and plant extract-mediated method. The influence of microwave irradiation was studied in both cases. The size, composition, crystallinity and morphology of inorganic nanoparticles (NPs) were investigated using dynamic light scattering (DLS), powder X-ray diffraction (XRD), SEM-EDX microscopy. Tunings of the nanochemistry reaction conditions (Zn precursor, structuring agent), ZnO NPs with various shapes were obtained, from quasi-spherical to flower-like. The optical properties and photocatalytic activity (degradation of methylene blue as model compound) were also investigated. ZnO nanopowders' antibacterial activity was tested against Gram-positive and Gram-negative bacterial strains to evidence the influence of the vegetal extract-mediated synthesis on the biological activity.
Keywords: ZnO nanoparticles; biocompatible photocatalysts; green synthesis; microwave synthesis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures





References
-
- Zhang Y.-H., Li Y.-L., Gong F.-L., Xie K.-F., Liu M., Zhang H.-L., Fang S.-M. Al Doped Narcissus-like ZnO for Enhanced NO2 Sensing Performance: An Experimental and DFT Investigation. Sens. Actuators B Chem. 2020;305:127489. doi: 10.1016/j.snb.2019.127489. - DOI
-
- Mirzaei H., Darroudi M. Zinc Oxide Nanoparticles: Biological Synthesis and Biomedical Applications. Ceram. Int. 2017;43:907–914. doi: 10.1016/j.ceramint.2016.10.051. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

