Effect of D-Mannitol on the Microstructure and Rheology of Non-Aqueous Carbopol Microgels
- PMID: 33916550
- PMCID: PMC8038445
- DOI: 10.3390/ma14071782
Effect of D-Mannitol on the Microstructure and Rheology of Non-Aqueous Carbopol Microgels
Abstract
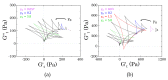
D-mannitol is a common polyol that is used as additive in pharmaceutical and personal care product formulations. We investigated its effect on the microstructure and rheology of novel non-aqueous Carbopol dispersions employing traditional and time-resolved rheological analysis. We considered two types of sample, (i) fresh (i.e., mannitol completely dissolved in solution) and aged (i.e., visible in crystalline form). The analysis of the intracycle rheological transitions that were observed for different samples revealed that, when completely dissolved in solution, mannitol does not alter the rheological behaviour of the Carbopol dispersions. This highlights that the chemical similarity of the additive with the molecules of the surrounding solvent allows preserving the swollen dimension and interparticle interactions of the Carbopol molecules. Conversely, when crystals are present, a hierarchical structure forms, consisting of a small dispersed phase (Carbopol) agglomerated around a big dispersed phase (crystals). In keeping with this microstructural picture, as the concentration of Carbopol reduces, the local dynamics of the crystals gradually start to control the integrity of the microstructure. Rheologically, this results in a higher elasticity of the suspensions at infinitesimal deformations, but a fragile yielding process at intermediate strains.
Keywords: carbopol; fluorescence microscopy; mannitol; microgels; non-aqueous formulations; nonlinear rheology, LAOS; structure-rheology relationship; yield stress.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures














References
-
- Karg M., Pich A., Hellweg T., Hoare T., Lyon L.A., Crassous J.J., Suzuki D., Gumerov R.A., Schneider S., Potemkin I.I., et al. Nanogels and Microgels: From Model Colloids to Applications, Recent Developments, and Future Trends. Langmuir. 2019;35:6231–6255. doi: 10.1021/acs.langmuir.8b04304. - DOI - PubMed
-
- Smeets N.M., Hoare T. Designing responsive microgels for drug delivery applications. J. Polym. Sci. Part Polym. Chem. 2013;51:3027–3043. doi: 10.1002/pola.26707. - DOI
-
- Torres O., Murray B., Sarkar A. Design of novel emulsion microgel particles of tuneable size. Food Hydrocoll. 2017;71:47–59. doi: 10.1016/j.foodhyd.2017.04.029. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

