Targeting Protein Kinase C in Glioblastoma Treatment
- PMID: 33916593
- PMCID: PMC8067000
- DOI: 10.3390/biomedicines9040381
Targeting Protein Kinase C in Glioblastoma Treatment
Abstract
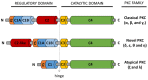
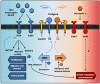
Glioblastoma (GBM) is the most frequent and aggressive primary brain tumor and is associated with a poor prognosis. Despite the use of combined treatment approaches, recurrence is almost inevitable and survival longer than 14 or 15 months after diagnosis is low. It is therefore necessary to identify new therapeutic targets to fight GBM progression and recurrence. Some publications have pointed out the role of glioma stem cells (GSCs) as the origin of GBM. These cells, with characteristics of neural stem cells (NSC) present in physiological neurogenic niches, have been proposed as being responsible for the high resistance of GBM to current treatments such as temozolomide (TMZ). The protein Kinase C (PKC) family members play an essential role in transducing signals related with cell cycle entrance, differentiation and apoptosis in NSC and participate in distinct signaling cascades that determine NSC and GSC dynamics. Thus, PKC could be a suitable druggable target to treat recurrent GBM. Clinical trials have tested the efficacy of PKCβ inhibitors, and preclinical studies have focused on other PKC isozymes. Here, we discuss the idea that other PKC isozymes may also be involved in GBM progression and that the development of a new generation of effective drugs should consider the balance between the activation of different PKC subtypes.
Keywords: enzastaurin; epidermal growth factor receptor; glioblastoma; glioma stem cells; neural stem cells; neuregulin; neurogenesis; protein kinase C; temozolomide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

