Enhanced Intestinal TGF-β/SMAD-Dependent Signaling in Simian Immunodeficiency Virus Infected Rhesus Macaques
- PMID: 33916615
- PMCID: PMC8066988
- DOI: 10.3390/cells10040806
Enhanced Intestinal TGF-β/SMAD-Dependent Signaling in Simian Immunodeficiency Virus Infected Rhesus Macaques
Abstract
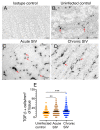
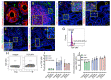
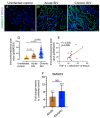
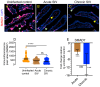
Transforming growth factor-β signaling (TGF-β) maintains a balanced physiological function including cell growth, differentiation, and proliferation and regulation of immune system by modulating either SMAD2/3 and SMAD7 (SMAD-dependent) or SMAD-independent signaling pathways under normal conditions. Increased production of TGF-β promotes immunosuppression in Human Immunodeficiency Virus (HIV)/Simian Immunodeficiency Virus (SIV) infection. However, the cellular source and downstream events of increased TGF-β production that attributes to its pathological manifestations remain unknown. Here, we have shown increased production of TGF-β in a majority of intestinal CD3-CD20-CD68+ cells from acute and chronically SIV infected rhesus macaques, which negatively correlated with the frequency of jejunum CD4+ T cells. No significant changes in intestinal TGF-β receptor II expression were observed but increased production of the pSMAD2/3 protein and SMAD3 gene expression in jejunum tissues that were accompanied by a downregulation of SMAD7 protein and gene expression. Enhanced TGF-β production by intestinal CD3-CD20-CD68+ cells and increased TGF-β/SMAD-dependent signaling might be due to a disruption of a negative feedback loop mediated by SMAD7. This suggests that SIV infection impacts the SMAD-dependent signaling pathway of TGF-β and provides a potential framework for further study to understand the role of viral factor(s) in modulating TGF-β production and downregulating SMAD7 expression in SIV. Regulation of mucosal TGF-β expression by therapeutic TGF-β blockers may help to create effective antiviral mucosal immune responses.
Keywords: CD4; CD68; SMAD signaling pathway; SMAD3; SMAD7; TGF-β receptor; TGF-β1; rhesus macaque; simian immunodeficiency virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Longitudinal Examination of the Intestinal Lamina Propria Cellular Compartment of Simian Immunodeficiency Virus-Infected Rhesus Macaques Provides Broader and Deeper Insights into the Link between Aberrant MicroRNA Expression and Persistent Immune Activation.J Virol. 2016 Apr 29;90(10):5003-5019. doi: 10.1128/JVI.00189-16. Print 2016 May 15. J Virol. 2016. PMID: 26937033 Free PMC article.
-
Simian Immunodeficiency Virus Infection Mediated Changes in Jejunum and Peripheral SARS-CoV-2 Receptor ACE2 and Associated Proteins or Genes in Rhesus Macaques.Front Immunol. 2022 Feb 25;13:835686. doi: 10.3389/fimmu.2022.835686. eCollection 2022. Front Immunol. 2022. PMID: 35281029 Free PMC article.
-
TGF-beta in intestinal lymphoid organs contributes to the death of armed effector CD8 T cells and is associated with the absence of virus containment in rhesus macaques infected with the simian immunodeficiency virus.Cell Death Differ. 2007 Oct;14(10):1747-58. doi: 10.1038/sj.cdd.4402192. Epub 2007 Jul 6. Cell Death Differ. 2007. PMID: 17612589
-
Central role of dysregulation of TGF-β/Smad in CKD progression and potential targets of its treatment.Biomed Pharmacother. 2018 May;101:670-681. doi: 10.1016/j.biopha.2018.02.090. Epub 2018 Mar 22. Biomed Pharmacother. 2018. PMID: 29518614 Review.
-
TGF-β/SMAD Pathway and Its Regulation in Hepatic Fibrosis.J Histochem Cytochem. 2016 Mar;64(3):157-67. doi: 10.1369/0022155415627681. Epub 2016 Jan 8. J Histochem Cytochem. 2016. PMID: 26747705 Free PMC article. Review.
Cited by
-
Divergent Cytokine and Chemokine Responses at Early Acute Simian Immunodeficiency Virus Infection Correlated with Virus Replication and CD4 T Cell Loss in a Rhesus Macaque Model.Vaccines (Basel). 2023 Jan 25;11(2):264. doi: 10.3390/vaccines11020264. Vaccines (Basel). 2023. PMID: 36851142 Free PMC article.
-
Identification, Characterization, and Transcriptional Reprogramming of Epithelial Stem Cells and Intestinal Enteroids in Simian Immunodeficiency Virus Infected Rhesus Macaques.Front Immunol. 2021 Nov 23;12:769990. doi: 10.3389/fimmu.2021.769990. eCollection 2021. Front Immunol. 2021. PMID: 34887863 Free PMC article.
-
Transcriptomic Analysis Reveals Key Pathways Influenced by HIV-2 Vpx.Int J Mol Sci. 2025 Apr 8;26(8):3460. doi: 10.3390/ijms26083460. Int J Mol Sci. 2025. PMID: 40331967 Free PMC article.
-
New Insights in the Fight against HIV.Cells. 2021 Dec 16;10(12):3549. doi: 10.3390/cells10123549. Cells. 2021. PMID: 34944057 Free PMC article.
-
Mycn regulates intestinal development through ribosomal biogenesis in a zebrafish model of Feingold syndrome 1.PLoS Biol. 2022 Nov 1;20(11):e3001856. doi: 10.1371/journal.pbio.3001856. eCollection 2022 Nov. PLoS Biol. 2022. PMID: 36318514 Free PMC article.
References
-
- Pahar B., Pan D., Lala W., Kenway-Lynch C.S., Das A. Transforming growth factor-β1 regulated phosphorylated AKT and interferon gamma expressions are associated with epithelial cell survival in rhesus macaque colon explants. Clin. Immunol. 2015;158:8–18. doi: 10.1016/j.clim.2015.03.001. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

