Can Vitamin B12 Assist the Internalization of Antisense LNA Oligonucleotides into Bacteria?
- PMID: 33916701
- PMCID: PMC8065541
- DOI: 10.3390/antibiotics10040379
Can Vitamin B12 Assist the Internalization of Antisense LNA Oligonucleotides into Bacteria?
Abstract
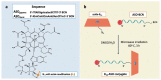
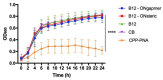
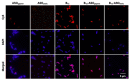
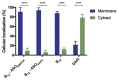
The emergence of bacterial resistance to traditional small-molecule antibiotics is fueling the search for innovative strategies to treat infections. Inhibiting the expression of essential bacterial genes using antisense oligonucleotides (ASOs), particularly composed of nucleic acid mimics (NAMs), has emerged as a promising strategy. However, their efficiency depends on their association with vectors that can translocate the bacterial envelope. Vitamin B12 is among the largest molecules known to be taken up by bacteria and has very recently started to gain interest as a trojan-horse vector. Gapmers and steric blockers were evaluated as ASOs against Escherichia coli (E. coli). Both ASOs were successfully conjugated to B12 by copper-free azide-alkyne click-chemistry. The biological effect of the two conjugates was evaluated together with their intracellular localization in E. coli. Although not only B12 but also both B12-ASO conjugates interacted strongly with E. coli, they were mostly colocalized with the outer membrane. Only 6-9% were detected in the cytosol, which showed to be insufficient for bacterial growth inhibition. These results suggest that the internalization of B12-ASO conjugates is strongly affected by the low uptake rate of the B12 in E. coli and that further studies are needed before considering this strategy against biofilms in vivo.
Keywords: 2′OMe; LNA; antibacterial drug; antisense oligonucleotides; nucleic acid mimics; vitamin B12.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- WHO . The Evolving Threat of Antimicrobial Resistance: Options for Action. World Health Organization; Geneva, Switzerland: 2012.
-
- Bai H., You Y., Yan H., Meng J., Xue X., Hou Z., Zhou Y., Ma X., Sang G., Luo X. Antisense inhibition of gene expression and growth in gram-negative bacteria by cell-penetrating peptide conjugates of peptide nucleic acids targeted to rpoD gene. Biomaterials. 2012;33:659–667. doi: 10.1016/j.biomaterials.2011.09.075. - DOI - PubMed
-
- Wengel J. Synthesis of 3 ‘-C-and 4 ‘-C-branched oligodeoxynucleotides and the development of locked nucleic acid (LNA) Acc. Chem. Res. 1999;32:301–310. doi: 10.1021/ar980051p. - DOI
Grants and funding
- SFRH/BD/118018/2016/Fundação para a Ciência e a Tecnologia
- Project UID/EQU/00511/2019 - Laboratory for Process Engineering, Environment, Biotechnology and Energy - LEPABE/FCT/MCTES (PIDDAC)
- 810685/European Union's Horizon 2020 research and innovation program
- Project "LEPABE-2-ECO-INNOVATION" - NORTE-01-0145-FEDER-000005/Norte Portugal Regional Operational Programme (NORTE 2020), under PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF)
- VKR18333/VILLUM FONDEN
LinkOut - more resources
Full Text Sources
Other Literature Sources

