Immunomodulatory Effects of Bendamustine in Hematopoietic Cell Transplantation
- PMID: 33916711
- PMCID: PMC8038415
- DOI: 10.3390/cancers13071702
Immunomodulatory Effects of Bendamustine in Hematopoietic Cell Transplantation
Abstract
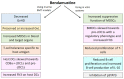
Bendamustine (BEN) is a unique alkylating agent with efficacy against a broad range of hematological malignancies, although investigations have only recently started to delve into its immunomodulatory effects. These immunomodulatory properties of BEN in the context of hematopoietic cell transplantation (HCT) are reviewed here. Pre- and post-transplant use of BEN in multiple murine models have consistently resulted in reduced GvHD and enhanced GvL, with significant changes to key immunological cell populations, including T-cells, myeloid derived suppressor cells (MDSCs), and dendritic cells (DCs). Further, in vitro studies find that BEN enhances the suppressive function of MDSCs, skews DCs toward cDC1s, enhances Flt3 expression on DCs, increases B-cell production of IL-10, inhibits STAT3 activation, and suppresses proliferation of T- and B-cells. Overall, BEN has a broad range of immunomodulatory effects that, as they are further elucidated, may be exploited to improve clinical outcomes. As such, clinical trials are currently underway investigating new potential applications of BEN in the setting of allogeneic HCT.
Keywords: bendamustine; hematopoietic cell transplantation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Bendamustine Conditioning Skews Murine Host DCs Toward Pre-cDC1s and Reduces GvHD Independently of Batf3.Front Immunol. 2020 Jul 16;11:1410. doi: 10.3389/fimmu.2020.01410. eCollection 2020. Front Immunol. 2020. PMID: 32765499 Free PMC article.
-
Regulatory Dendritic Cells Induced by Bendamustine Are Associated With Enhanced Flt3 Expression and Alloreactive T-Cell Death.Front Immunol. 2021 Jun 24;12:699128. doi: 10.3389/fimmu.2021.699128. eCollection 2021. Front Immunol. 2021. PMID: 34249005 Free PMC article.
-
Post-transplant bendamustine reduces GvHD while preserving GvL in experimental haploidentical bone marrow transplantation.Br J Haematol. 2016 Jul;174(1):102-16. doi: 10.1111/bjh.14034. Epub 2016 Mar 31. Br J Haematol. 2016. PMID: 27030315 Free PMC article.
-
The Role of Myeloid-Derived Suppressor Cells (MDSCs) in Graft-versus-Host Disease (GVHD).J Clin Med. 2021 May 11;10(10):2050. doi: 10.3390/jcm10102050. J Clin Med. 2021. PMID: 34064671 Free PMC article. Review.
-
Alloreactivity as therapeutic principle in the treatment of hematologic malignancies. Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning.Dan Med Bull. 2007 May;54(2):112-39. Dan Med Bull. 2007. PMID: 17521527 Review.
Cited by
-
Myeloid-derived suppressor cells in hematologic malignancies: two sides of the same coin.Exp Hematol Oncol. 2022 Jul 19;11(1):43. doi: 10.1186/s40164-022-00296-9. Exp Hematol Oncol. 2022. PMID: 35854339 Free PMC article. Review.
-
Partially replacing cyclophosphamide with bendamustine in combination with cyclosporine A improves survival and reduces xenogeneic graft-versus-host-disease.Front Immunol. 2023 Jan 9;13:1045710. doi: 10.3389/fimmu.2022.1045710. eCollection 2022. Front Immunol. 2023. PMID: 36700195 Free PMC article.
-
Feasibility and efficacy of partial replacement of post transplantation cyclophosphamide with bendamustine on day +4 for graft versus host disease prophylaxis in patients undergoing allogeneic hematopoietic cell transplantation.Bone Marrow Transplant. 2025 Jul;60(7):994-1001. doi: 10.1038/s41409-025-02581-7. Epub 2025 Apr 21. Bone Marrow Transplant. 2025. PMID: 40258995
-
Feasibility and Efficacy of Partially Replacing Post-Transplantation Cyclophosphamide with Bendamustine in Pediatric and Young Adult Patients Undergoing Haploidentical Bone Marrow Transplantation.Transplant Cell Ther. 2022 Jul;28(7):390.e1-390.e10. doi: 10.1016/j.jtct.2022.04.015. Epub 2022 Apr 20. Transplant Cell Ther. 2022. PMID: 35460929 Free PMC article. Clinical Trial.
-
Commentary: Post-Transplantation Cyclophosphamide Uniquely Restrains Alloreactive CD4+ T-Cell Proliferation and Differentiation After Murine MHC-Haploidentical Hematopoietic Cell Transplantation.Front Immunol. 2022 Apr 14;13:887648. doi: 10.3389/fimmu.2022.887648. eCollection 2022. Front Immunol. 2022. PMID: 35493453 Free PMC article. No abstract available.
References
-
- Cephalon Cephalon Receives FDA Approval for TREANDA, a Novel Chemotherapy for Chronic Lymphocytic Leukemia. [(accessed on 21 October 2017)];2008 Available online: www.cephalon.com.
-
- Leoni L.M., Bailey B., Reifert J., Bendall H.H., Zeller R.W., Corbeil J., Elliott G., Niemeyer C.C. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008;14:309–317. doi: 10.1158/1078-0432.CCR-07-1061. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous