Repurposing Avermectins and Milbemycins against Mycobacteroides abscessus and Other Nontuberculous Mycobacteria
- PMID: 33916775
- PMCID: PMC8066277
- DOI: 10.3390/antibiotics10040381
Repurposing Avermectins and Milbemycins against Mycobacteroides abscessus and Other Nontuberculous Mycobacteria
Abstract
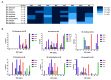
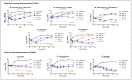
Infections caused by nontuberculous mycobacteria (NTM) are increasing worldwide, resulting in a new global health concern. NTM treatment is complex and requires combinations of several drugs for lengthy periods. In spite of this, NTM disease is often associated with poor treatment outcomes. The anti-parasitic family of macrocyclic lactones (ML) (divided in two subfamilies: avermectins and milbemycins) was previously described as having activity against mycobacteria, including Mycobacterium tuberculosis, Mycobacterium ulcerans, and Mycobacterium marinum, among others. Here, we aimed to characterize the in vitro anti-mycobacterial activity of ML against a wide range of NTM species, including Mycobacteroides abscessus. For this, Minimum Inhibitory Concentration (MIC) values of eight ML were determined against 80 strains belonging to nine different NTM species. Macrocyclic lactones showed variable ranges of anti-mycobacterial activity that were compound and species-dependent. Milbemycin oxime was the most active compound, displaying broad-spectrum activity with MIC lower than 8 mg/L. Time kill assays confirmed MIC data and showed bactericidal and sterilizing activity of some compounds. Macrocyclic lactones are available in many formulations and have been extensively used in veterinary and human medicine with suitable pharmacokinetics and safety properties. This information could be exploited to explore repurposing of anti-helminthics for NTM therapy.
Keywords: Mycobacteroides abscessus; avermectins; milbemycin oxime; nontuberculous mycobacteria; repurposing; selamectin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Selamectin Is the Avermectin with the Best Potential for Buruli Ulcer Treatment.PLoS Negl Trop Dis. 2015 Aug 13;9(8):e0003996. doi: 10.1371/journal.pntd.0003996. eCollection 2015 Aug. PLoS Negl Trop Dis. 2015. PMID: 26270480 Free PMC article.
-
The emergence of resistance to the antiparasitic selamectin in Mycobacterium smegmatis is improbable and contingent on cell wall integrity.Microbiol Spectr. 2025 Apr 8;13(5):e0233224. doi: 10.1128/spectrum.02332-24. Online ahead of print. Microbiol Spectr. 2025. PMID: 40197087 Free PMC article.
-
In Vitro Activity of Bedaquiline and Delamanid against Nontuberculous Mycobacteria, Including Macrolide-Resistant Clinical Isolates.Antimicrob Agents Chemother. 2019 Jul 25;63(8):e00665-19. doi: 10.1128/AAC.00665-19. Print 2019 Aug. Antimicrob Agents Chemother. 2019. PMID: 31182533 Free PMC article.
-
Host-Pathogen Interactions Operative during Mycobacteroides abscessus Infection.Immune Netw. 2021 Dec 23;21(6):e40. doi: 10.4110/in.2021.21.e40. eCollection 2021 Dec. Immune Netw. 2021. PMID: 35036027 Free PMC article. Review.
-
Recent advances in molecular diagnostics and understanding mechanisms of drug resistance in nontuberculous mycobacterial diseases.Infect Genet Evol. 2019 Aug;72:169-182. doi: 10.1016/j.meegid.2018.10.003. Epub 2018 Oct 11. Infect Genet Evol. 2019. PMID: 30315892 Review.
Cited by
-
Repurposing β-Lactams for the Treatment of Mycobacterium kansasii Infections: An In Vitro Study.Antibiotics (Basel). 2023 Feb 5;12(2):335. doi: 10.3390/antibiotics12020335. Antibiotics (Basel). 2023. PMID: 36830246 Free PMC article.
-
The Veterinary Anti-Parasitic Selamectin Is a Novel Inhibitor of the Mycobacterium tuberculosis DprE1 Enzyme.Int J Mol Sci. 2022 Jan 11;23(2):771. doi: 10.3390/ijms23020771. Int J Mol Sci. 2022. PMID: 35054958 Free PMC article.
-
Repurposing Molnupiravir for COVID-19: The Mechanisms of Antiviral Activity.Viruses. 2022 Jun 20;14(6):1345. doi: 10.3390/v14061345. Viruses. 2022. PMID: 35746815 Free PMC article. Review.
-
Exploring novel microbial metabolites and drugs for inhibiting Clostridioides difficile.mSphere. 2024 Jul 30;9(7):e0027324. doi: 10.1128/msphere.00273-24. Epub 2024 Jun 28. mSphere. 2024. PMID: 38940508 Free PMC article.
-
Ivermectin Attenuates CCl4-Induced Liver Fibrosis in Mice by Suppressing Hepatic Stellate Cell Activation.Int J Mol Sci. 2022 Dec 16;23(24):16043. doi: 10.3390/ijms232416043. Int J Mol Sci. 2022. PMID: 36555680 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

