Prevalence of Worldwide Neonatal Calf Diarrhoea Caused by Bovine Rotavirus in Combination with Bovine Coronavirus, Escherichia coli K99 and Cryptosporidium spp.: A Meta-Analysis
- PMID: 33916839
- PMCID: PMC8066230
- DOI: 10.3390/ani11041014
Prevalence of Worldwide Neonatal Calf Diarrhoea Caused by Bovine Rotavirus in Combination with Bovine Coronavirus, Escherichia coli K99 and Cryptosporidium spp.: A Meta-Analysis
Abstract
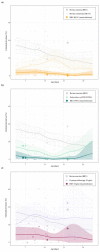
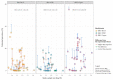
Multiple enteropathogens such as bovine rotavirus (BRV), bovine coronavirus (BCoV), Escherichia coli K99 (ETEC) and Cryptosporidium spp. (Crypto) are the most common causes of calf diarrhoea during the first 30 days of animal age. Three weighted-stratified random-effects meta-analyses were performed to calculate the worldwide prevalence of mixed infections of the causative agents (i.e., BRV-BCoV, BRV-ETEC, BRV-Crypto) and their potential influencing factors. The meta-analysis covered 41 studies (94 sub-studies) in 21 countries that determined the presence or absence of mixed infections in global calf populations. The highest worldwide estimated pooled prevalence was identified for BRV-Crypto (6.69%), followed by BRV-BCoV (2.84%), and BRV-ETEC (1.64%). The chance of detecting BCoV in calves with diarrhoea was 1.83 higher in the presence of BRV compared to calves without BRV, whereby an inhibition effect (odds ratio: 0.77) was determined between BRV and Crypto infections. The diagnostic methods were identified as a significant influencing factor in the detection of all considered mixed infections, while the other analysed factors differed in relation to their effect on prevalence. In contrast to BRV-BCoV, the prevalence of BRV-ETEC and BRV-Crypto mixed infections followed the course of individual ETEC and Crypto prevalence related to the age class of the sampled animals.
Keywords: Cryptosporidium spp.; Escherichia coli K99; bovine coronavirus; bovine rotavirus; concurrent-infection; epidemiology; mixed-infection; pathogens; systematic review.
Conflict of interest statement
The authors declare no conflict of interest. None of the authors of this paper has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
Figures

References
-
- Pinior B., Firth C.L., Richter V., Lebl K., Trauffler M., Dzieciol M., Hutter S.E., Burgstaller J., Obritzhauser W., Winter P., et al. A systematic review of financial and economic assessments of bovine viral diarrhea virus (BVDV) prevention and mitigation activities worldwide. Prev. Vet. Med. 2017;137:77–92. doi: 10.1016/j.prevetmed.2016.12.014. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

