Gingival Crevicular Placental Alkaline Phosphatase Is an Early Pregnancy Biomarker for Pre-Eclampsia
- PMID: 33916883
- PMCID: PMC8067553
- DOI: 10.3390/diagnostics11040661
Gingival Crevicular Placental Alkaline Phosphatase Is an Early Pregnancy Biomarker for Pre-Eclampsia
Abstract
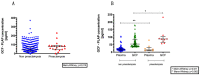
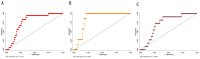
Early and innovative diagnostic strategies are required to predict the risk of developing pre-eclampsia (PE). The purpose of this study was to evaluate the performance of gingival crevicular fluid (GCF) placental alkaline phosphatase (PLAP) concentrations to correctly classify women at risk of PE. A prospectively collected, retrospectively stratified cohort study was conducted, with 412 pregnant women recruited at 11-14 weeks of gestation. Physical, obstetrical, and periodontal data were recorded. GCF and blood samples were collected for PLAP determination by ELISA assay. A multiple logistic regression classification model was developed, and the classification efficiency of the model was established. Within the study cohort, 4.3% of pregnancies developed PE. GCF-PLAP concentration was 3- to 6-fold higher than in plasma samples. GCF-PLAP concentrations and systolic blood pressure were greater in women who developed PE (p = 0.015 and p < 0.001, respectively). The performance of the multiparametric model that combines GCF-PLAP concentration and the levels of systolic blood pressure (at 11-14 weeks gestation) showed an association of systolic blood pressure and GCF-PLAP concentrations with the likelihood of developing PE (OR:1.07; 95% CI 1.01-1.11; p = 0.004 and OR:1.008, 95% CI 1.000-1.015; p = 0.034, respectively). The model had a sensitivity of 83%, a specificity of 72%, and positive and negative predictive values of 12% and 99%, respectively. The area under the receiver operating characteristic (AUC-ROC) curve was 0.77 and correctly classified 72% of PE pregnancies. In conclusion, the multivariate classification model developed may be of utility as an aid in identifying pre-symptomatic women who subsequently develop PE.
Keywords: cohort study; gestation; placental biomarkers; pre-eclampsia; risk prediction model.
Conflict of interest statement
The authors have stated explicitly that there are no conflicts of interest concerning this manuscript. The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Figures
Similar articles
-
The potential role of the gingival crevicular fluid biomarkers in the prediction of pregnancy complications.Front Med (Lausanne). 2023 Jun 5;10:1168625. doi: 10.3389/fmed.2023.1168625. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37342498 Free PMC article. Review.
-
Periodontitis and placental growth factor in oral fluids are early pregnancy predictors of gestational diabetes mellitus.J Periodontol. 2018 Sep;89(9):1052-1060. doi: 10.1002/JPER.17-0497. J Periodontol. 2018. PMID: 29790168
-
Placental biomarkers and angiogenic factors in oral fluids of patients with preeclampsia.Prenat Diagn. 2016 May;36(5):476-82. doi: 10.1002/pd.4811. Epub 2016 Apr 7. Prenat Diagn. 2016. PMID: 26988336
-
Prediction of pre-eclampsia and its subtypes in high-risk cohort: hyperglycosylated human chorionic gonadotropin in multivariate models.BMC Pregnancy Childbirth. 2018 Jul 3;18(1):279. doi: 10.1186/s12884-018-1908-9. BMC Pregnancy Childbirth. 2018. PMID: 29970026 Free PMC article. Clinical Trial.
-
First-trimester prediction of pre-eclampsia: external validity of algorithms in a prospectively enrolled cohort.Ultrasound Obstet Gynecol. 2014 Sep;44(3):279-85. doi: 10.1002/uog.13435. Epub 2014 Aug 13. Ultrasound Obstet Gynecol. 2014. PMID: 24913190 Review.
Cited by
-
The potential role of the gingival crevicular fluid biomarkers in the prediction of pregnancy complications.Front Med (Lausanne). 2023 Jun 5;10:1168625. doi: 10.3389/fmed.2023.1168625. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37342498 Free PMC article. Review.
-
Obesity is related to maternal periodontitis severity in pregnancy: a cross-sectional study.Clin Oral Investig. 2023 Sep;27(9):5509-5518. doi: 10.1007/s00784-023-05170-4. Epub 2023 Jul 28. Clin Oral Investig. 2023. PMID: 37505241
-
Toward a new taxonomy of obstetrical disease: improved performance of maternal blood biomarkers for the great obstetrical syndromes when classified according to placental pathology.Am J Obstet Gynecol. 2022 Oct;227(4):615.e1-615.e25. doi: 10.1016/j.ajog.2022.04.015. Epub 2022 Sep 3. Am J Obstet Gynecol. 2022. PMID: 36180175 Free PMC article.
-
Alkaline phosphatase of late pregnancy promotes the prediction of adverse birth outcomes.J Glob Health. 2025 Jan 24;15:04028. doi: 10.7189/jogh.15.04028. J Glob Health. 2025. PMID: 39849974 Free PMC article.
References
-
- Magee L.A., Pels A., Helewa M., Rey E., von Dadelszen P., Audibert F., Bujold E., Côté A.-M., Douglas M.J., Eastabrook G., et al. Diagnosis, Evaluation, and Management of the Hypertensive Disorders of Pregnancy: Executive Summary. J. Obstet. Gynaecol. Can. 2014;36:416–438. doi: 10.1016/S1701-2163(15)30588-0. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

