Rhinovirus and Cell Death
- PMID: 33916958
- PMCID: PMC8067602
- DOI: 10.3390/v13040629
Rhinovirus and Cell Death
Abstract
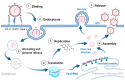
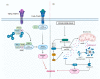
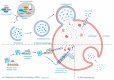
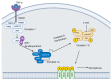
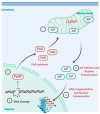
Rhinoviruses (RVs) are the etiological agents of upper respiratory tract infections, particularly the common cold. Infections in the lower respiratory tract is shown to cause severe disease and exacerbations in asthma and COPD patients. Viruses being obligate parasites, hijack host cell pathways such as programmed cell death to suppress host antiviral responses and prolong viral replication and propagation. RVs are non-enveloped positive sense RNA viruses with a lifecycle fully contained within the cytoplasm. Despite decades of study, the details of how RVs exit the infected cell are still unclear. There are some diverse studies that suggest a possible role for programmed cell death. In this review, we aimed to consolidate current literature on the impact of RVs on cell death to inform future research on the topic. We searched peer reviewed English language literature in the past 21 years for studies on the interaction with and modulation of cell death pathways by RVs, placing it in the context of the broader knowledge of these interconnected pathways from other systems. Our review strongly suggests a role for necroptosis and/or autophagy in RV release, with the caveat that all the literature is based on RV-A and RV-B strains, with no studies to date examining the interaction of RV-C strains with cell death pathways.
Keywords: apoptosis; autophagy; cell death pathways; lifecycle; necroptosis; necrosis; rhinovirus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Kuo C.-Y., Chiu V., Hsieh P.-C., Huang C.-Y., Huang S.J., Tzeng I.S., Tsai F.-M., Chen M.-L., Liu C.-T., Chen Y.-R. Chrysophanol attenuates hepatitis B virus X protein-induced hepatic stellate cell fibrosis by regulating endoplasmic reticulum stress and ferroptosis. J. Pharmacol. Sci. 2020;144:172–182. doi: 10.1016/j.jphs.2020.07.014. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

