Novel Pyrimidine Derivatives as Potential Anticancer Agents: Synthesis, Biological Evaluation and Molecular Docking Study
- PMID: 33917090
- PMCID: PMC8067809
- DOI: 10.3390/ijms22083825
Novel Pyrimidine Derivatives as Potential Anticancer Agents: Synthesis, Biological Evaluation and Molecular Docking Study
Abstract
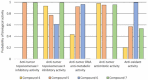
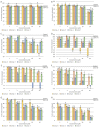
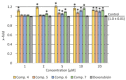
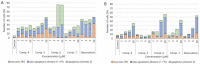
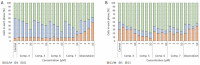
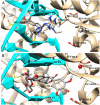
In the present paper, new pyrimidine derivatives were designed, synthesized and analyzed in terms of their anticancer properties. The tested compounds were evaluated in vitro for their antitumor activity. The cytotoxic effect on normal human dermal fibroblasts (NHDF) was also determined. According to the results, all the tested compounds exhibited inhibitory activity on the proliferation of all lines of cancer cells (colon adenocarcinoma (LoVo), resistant colon adenocarcinoma (LoVo/DX), breast cancer (MCF-7), lung cancer (A549), cervical cancer (HeLa), human leukemic lymphoblasts (CCRF-CEM) and human monocytic (THP-1)). In particular, their feature stronger influence on the activity of P-glycoprotein of cell cultures resistant to doxorubicin than doxorubicin. Tested compounds have more lipophilic character than doxorubicin, which determines their affinity for the molecular target and passive transport through biological membranes. Moreover, the inhibitory potential against topoisomerase II and DNA intercalating properties of synthesized compounds were analyzed via molecular docking.
Keywords: 3,4-dihydronaphthalen; 6-hydrazinopyrimidine; DNA intercalating; QSAR study; anticancer; lipophilicity; pyrimidine; topoisomerase II.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Su L., Li J., Zhou Z., Huang D., Zhang Y., Pei H., Guo W., Wu H., Wang X., Liu M., et al. Corrigendum to “Design, synthesis and evaluation of hybrid of tetrahydrocarbazole with 2,4-diaminopyrimidine scaffold as antibacterial agents” [Eur. J. Med. Chem. 162 (162) (2019) 203–211] Eur. J. Med. Chem. 2019;168:385. doi: 10.1016/j.ejmech.2019.02.059. - DOI - PubMed
-
- Barakat A., Soliman S.M., Al-Majid A.M., Lotfy G., Ghabbour H.A., Fun H.K., Yousuf S., Choudhary M.I., Wadood A. Synthesis and structure investigation of novel pyrimidine-2,4,6-trione derivatives of highly potential biological activity as anti-diabetic agent. J. Mol. Struct. 2015;1098:365–376. doi: 10.1016/j.molstruc.2015.06.037. - DOI
-
- Zimmermann J. Pyrimidine Derivatives and Processes for the Preparation Thereof. US5521184A. U.S. Patent. 1996 May 28;
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

