Concomitant Swine Influenza A Virus Infection Alters PRRSV1 MLV Viremia in Piglets but Does Not Interfere with Vaccine Protection in Experimental Conditions
- PMID: 33917103
- PMCID: PMC8067798
- DOI: 10.3390/vaccines9040356
Concomitant Swine Influenza A Virus Infection Alters PRRSV1 MLV Viremia in Piglets but Does Not Interfere with Vaccine Protection in Experimental Conditions
Abstract
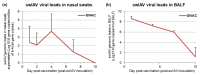
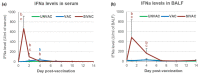
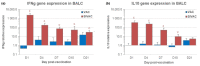
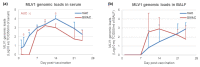
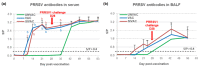
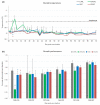
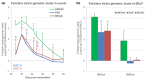
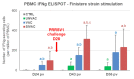
Modified-live vaccines (MLVs) against porcine reproductive and respiratory syndrome viruses (PRRSVs) are usually administrated to piglets at weaning when swine influenza A virus (swIAV) infections frequently occur. SwIAV infection induces a strong interferon alpha (IFNa) response and IFNa was shown to abrogate PRRSV2 MLV replication and an inherent immune response. In this study, we evaluated the impacts of swIAV infection on the replication of a PRRSV1 MLV (MLV1), post-vaccine immune responses and post-challenge vaccine efficacy at both the systemic and pulmonary levels. Piglets were either swIAV inoculated and MLV1 vaccinated 6 h apart or singly vaccinated or mock inoculated and mock vaccinated. Four weeks after vaccination, the piglets were challenged with a PRRSV1 field strain. The results showed that swIAV infection delayed MLV1 viremia by six days and post-vaccine seroconversion by four days. After the PRRSV1 challenge, the swIAV enhanced the PRRSV1-specific cell-mediated immunity (CMI) but the PRRSV1 field strain viremia was not better controlled. High IFNa levels that were detected early after swIAV infection could have been responsible for both the inhibition of MLV1 replication and CMI enhancement. Thus, whereas swIAV infection had a negative impact on humoral responses post-vaccination, it did not interfere with the protective effectiveness of the PRRSV MLV1 in our experimental conditions.
Keywords: interference; interferon; modified-live vaccine; porcine reproductive and respiratory syndrome; swine influenza A virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Siddell S.G., Walker P.J., Lefkowitz E.J., Mushegian A.R., Adams M.J., Dutilh B.E., Gorbalenya A.E., Harrach B., Harrison R.L., Junglen S., et al. Additional changes to taxonomy ratified in a special vote by the International Committee on Taxonomy of Viruses (October 2018) Arch. Virol. 2019;164:943–946. doi: 10.1007/s00705-018-04136-2. - DOI - PubMed
-
- Martelli P., Gozio S., Ferrari L., Rosina S., De Angelis E., Quintavalla C., Bottarelli E., Borghetti P. Efficacy of a modified live porcine reproductive and respiratory syndrome virus (PRRSV) vaccine in pigs naturally exposed to a heterologous European (Italian cluster) field strain: Clinical protection and cell-mediated immunity. Vaccine. 2009;27:3788–3799. doi: 10.1016/j.vaccine.2009.03.028. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

