Probiotics Supplements Reduce ER Stress and Gut Inflammation Associated with Gliadin Intake in a Mouse Model of Gluten Sensitivity
- PMID: 33917155
- PMCID: PMC8067866
- DOI: 10.3390/nu13041221
Probiotics Supplements Reduce ER Stress and Gut Inflammation Associated with Gliadin Intake in a Mouse Model of Gluten Sensitivity
Abstract
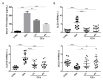
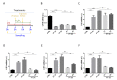
Exposure to gluten, a protein present in wheat rye and barley, is the major inducer for human Celiac Disease (CD), a chronic autoimmune enteropathy. CD occurs in about 1% worldwide population, in genetically predisposed individuals bearing human leukocyte antigen (HLA) DQ2/DQ8. Gut epithelial cell stress and the innate immune activation are responsible for the breaking oral tolerance to gliadin, a gluten component. To date, the only treatment available for CD is a long-term gluten-free diet. Several studies have shown that an altered composition of the intestinal microbiota (dysbiosis) could play a key role in the pathogenesis of CD through the modulation of intestinal permeability and the regulation of the immune system. Here, we show that gliadin induces a chronic endoplasmic reticulum (ER) stress condition in the small intestine of a gluten-sensitive mouse model and that the coadministration of probiotics efficiently attenuates both the unfolded protein response (UPR) and gut inflammation. Moreover, the composition of probiotics formulations might differ in their activity at molecular level, especially toward the three axes of the UPR. Therefore, probiotics administration might potentially represent a new valuable strategy to treat gluten-sensitive patients, such as those affected by CD.
Keywords: CD; CFTR; TG2; UPR; probiotics.
Conflict of interest statement
A.A. and M.P. are employees of Probiotical Research srl and have the following competing interests: probiotics production. They have no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Figures



References
-
- Molberg Ø., McAdam S.N., Körner R., Quarsten H., Kristiansen C., Madsen L., Fugger L., Scott H., Norén O., Roepstorff P., et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat. Med. 1998;4:713–717. doi: 10.1038/nm0698-713. - DOI - PubMed
-
- Bergseng E., Dørum S., Arntzen M.Ø., Nielsen M., Nygård S., Buus S., De Souza G.A., Sollid L.M. Different binding motifs of the celiac disease-associated HLA molecules DQ2.5, DQ2.2, and DQ7.5 revealed by relative quantitative proteomics of endogenous peptide repertoires. Immunogenetics. 2015;67:73–84. doi: 10.1007/s00251-014-0819-9. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

