Effect of Mycophenolate Mofetil Therapy on Recurrence of Hepatocellular Carcinoma after Liver Transplantation: A Population-Based Cohort Study
- PMID: 33917215
- PMCID: PMC8068064
- DOI: 10.3390/jcm10081558
Effect of Mycophenolate Mofetil Therapy on Recurrence of Hepatocellular Carcinoma after Liver Transplantation: A Population-Based Cohort Study
Abstract
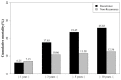
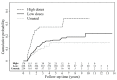
Hepatocellular carcinoma (HCC) recurrence after liver transplantation is associated with immunosuppressants. However, the appropriate immunosuppressant for HCC recipients is still debated. Data for this nationwide population-based cohort study were extracted from the National Health Insurance Research Database of Taiwan. A total of 1250 liver transplant recipients (LTRs) with HCC were included. We analyzed the risk factors for post-transplant HCC recurrences. Cumulative defined daily dose (cDDD) represented the exposure duration and was calculated as the amount of dispensed defined daily dose (DDD) of mycophenolate mofetil (MMF). The dosage effects of MMF on HCC recurrence and liver graft complication rates were investigated. A total of 155 LTRs, having experienced post-transplant HCC recurrence, exhibited low survival probability at 1-, 3-, 5-, and 10-year observations. Our results demonstrated increased HCC recurrence rate after liver transplantation (p = 0.0316) following MMF administration; however, no significant increase was demonstrated following cyclosporine, tacrolimus, or sirolimus administration. Notably, our data demonstrated significantly increased HCC recurrence rate following MMF administration with cDDD > 0.4893 compared with cDDD ≤ 0.4893 or no administration of MMF (p < 0.0001). MMF administration significantly increases the risk of HCC recurrence. Moreover, a MMF-minimizing strategy (cDDD ≤ 0.4893) is recommended for recurrence-free survival.
Keywords: hepatocellular carcinoma; immunosuppressant; liver transplantation; mycophenolate mofetil; population-based study; recommended defined daily dose; recurrence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Nanoparticle formulation of mycophenolate mofetil achieves enhanced efficacy against hepatocellular carcinoma by targeting tumour-associated fibroblast.J Cell Mol Med. 2021 Apr;25(7):3511-3523. doi: 10.1111/jcmm.16434. Epub 2021 Mar 13. J Cell Mol Med. 2021. PMID: 33713546 Free PMC article.
-
Tailored long-term immunosuppressive regimen for adult liver transplant recipients with hepatocellular carcinoma.Korean J Hepatobiliary Pancreat Surg. 2014 May;18(2):48-51. doi: 10.14701/kjhbps.2014.18.2.48. Epub 2014 May 31. Korean J Hepatobiliary Pancreat Surg. 2014. PMID: 26155248 Free PMC article.
-
Randomized, multicenter trial comparing tacrolimus plus mycophenolate mofetil to tacrolimus plus steroids in hepatitis C virus-positive recipients of living donor liver transplantation.Liver Transpl. 2013 Aug;19(8):896-906. doi: 10.1002/lt.23679. Liver Transpl. 2013. PMID: 23696054 Clinical Trial.
-
Mycophenolate, clinical pharmacokinetics, formulations, and methods for assessing drug exposure.Transplant Rev (Orlando). 2011 Apr;25(2):47-57. doi: 10.1016/j.trre.2010.06.001. Epub 2010 Dec 28. Transplant Rev (Orlando). 2011. PMID: 21190834
-
Systematic review with meta-analysis: sirolimus- or everolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma.Aliment Pharmacol Ther. 2019 May;49(10):1260-1273. doi: 10.1111/apt.15253. Epub 2019 Apr 15. Aliment Pharmacol Ther. 2019. PMID: 30989721
Cited by
-
Basic Understanding of Liver Transplant Immunology.J Clin Exp Hepatol. 2023 Nov-Dec;13(6):1091-1102. doi: 10.1016/j.jceh.2023.05.007. Epub 2023 May 26. J Clin Exp Hepatol. 2023. PMID: 37975047 Free PMC article. Review.
-
Challenges in Liver Transplantation for Hepatocellular Carcinoma: A Review of Current Controversies.Cancers (Basel). 2024 Sep 2;16(17):3059. doi: 10.3390/cancers16173059. Cancers (Basel). 2024. PMID: 39272917 Free PMC article. Review.
-
Immunotherapy for hepatocellular carcinoma recurrence after liver transplantation, can we harness the power of immune checkpoint inhibitors?Front Immunol. 2023 Feb 16;14:1092401. doi: 10.3389/fimmu.2023.1092401. eCollection 2023. Front Immunol. 2023. PMID: 36875077 Free PMC article. Review.
-
Identification of anoikis-related subtypes and a risk score prognosis model, the association with TME landscapes and therapeutic responses in hepatocellular carcinoma.Front Immunol. 2025 Jun 17;16:1602831. doi: 10.3389/fimmu.2025.1602831. eCollection 2025. Front Immunol. 2025. PMID: 40599775 Free PMC article.
References
-
- International Agency for Research on Cancer Liver. World Health Organization. [(accessed on 2 March 2020)];2018 Available online: http://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf.
-
- Mazzaferro V., Regalia E., Doci R., Andreola S., Pulvirenti A., Bozzetti F., Montalto F., Ammatuna M., Morabito A., Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N. Engl. J. Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. - DOI - PubMed
-
- Lai Q., Vitale A., Iesari S., Finkenstedt A., Mennini G., Spoletini G., Hoppe-Lotichius M., Vennarecci G., Manzia T.M., Nicolini D., et al. Intention-to-treat survival benefit of liver transplantation in patients with hepatocellular cancer. Hepatology. 2017;66:1910–1919. doi: 10.1002/hep.29342. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

