Traumatic Stress, Chronic Ethanol Exposure, or the Combination, Alter Cannabinoid System Components in Reward and Limbic Regions of the Mouse Brain
- PMID: 33917316
- PMCID: PMC8038692
- DOI: 10.3390/molecules26072086
Traumatic Stress, Chronic Ethanol Exposure, or the Combination, Alter Cannabinoid System Components in Reward and Limbic Regions of the Mouse Brain
Abstract
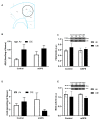
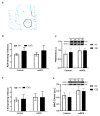
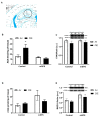
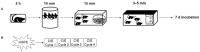
The cannabinoid system is independently affected by stress and chronic ethanol exposure. However, the extent to which co-occurrence of traumatic stress and chronic ethanol exposure modulates the cannabinoid system remains unclear. We examined levels of cannabinoid system components, anandamide, 2-arachidonoylglycerol, fatty acid amide hydrolase, and monoacylglycerol lipase after mouse single-prolonged stress (mSPS) or non-mSPS (Control) exposure, with chronic intermittent ethanol (CIE) vapor or without CIE vapor (Air) across several brain regions using ultra-high-performance liquid chromatography tandem mass spectrometry or immunoblotting. Compared to mSPS-Air mice, anandamide and 2-arachidonoylglycerol levels in the anterior striatum were increased in mSPS-CIE mice. In the dorsal hippocampus, anandamide content was increased in Control-CIE mice compared to Control-Air, mSPS-Air, or mSPS-CIE mice. Finally, amygdalar anandamide content was increased in Control-CIE mice compared to Control-Air, or mSPS-CIE mice, but the anandamide content was decreased in mSPS-CIE compared to mSPS-Air mice. Based on these data we conclude that the effects of combined traumatic stress and chronic ethanol exposure on the cannabinoid system in reward pathway regions are driven by CIE exposure and that traumatic stress affects the cannabinoid components in limbic regions, warranting future investigation of neurotherapeutic treatment to attenuate these effects.
Keywords: 2-arachidonoylglycerol; anandamide; chronic intermittent ethanol; fatty acid amide hydrolase; limbic system; monoacylglycerol lipase; mouse single-prolonged stress; post-traumatic stress disorder; reward pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Chronic Intermittent Ethanol Exposure Increases Ethanol Consumption Following Traumatic Stress Exposure in Mice.Front Behav Neurosci. 2020 Jun 30;14:114. doi: 10.3389/fnbeh.2020.00114. eCollection 2020. Front Behav Neurosci. 2020. PMID: 32694985 Free PMC article.
-
Alterations in endocannabinoid tone following chemotherapy-induced peripheral neuropathy: effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following cisplatin treatment.Pharmacol Res. 2013 Jan;67(1):94-109. doi: 10.1016/j.phrs.2012.10.013. Epub 2012 Nov 2. Pharmacol Res. 2013. PMID: 23127915 Free PMC article.
-
Guineensine is a novel inhibitor of endocannabinoid uptake showing cannabimimetic behavioral effects in BALB/c mice.Pharmacol Res. 2014 Feb;80:52-65. doi: 10.1016/j.phrs.2013.12.010. Epub 2014 Jan 8. Pharmacol Res. 2014. PMID: 24412246
-
Conformational requirements for endocannabinoid interaction with the cannabinoid receptors, the anandamide transporter and fatty acid amidohydrolase.Chem Phys Lipids. 2000 Nov;108(1-2):15-35. doi: 10.1016/s0009-3084(00)00185-7. Chem Phys Lipids. 2000. PMID: 11106780 Review.
-
Untapped endocannabinoid pharmacological targets: Pipe dream or pipeline?Pharmacol Biochem Behav. 2021 Jul;206:173192. doi: 10.1016/j.pbb.2021.173192. Epub 2021 Apr 29. Pharmacol Biochem Behav. 2021. PMID: 33932409 Review.
Cited by
-
Molecular Alterations of the Endocannabinoid System in Psychiatric Disorders.Int J Mol Sci. 2022 Apr 26;23(9):4764. doi: 10.3390/ijms23094764. Int J Mol Sci. 2022. PMID: 35563156 Free PMC article. Review.
-
Repeated Restraint Stress and Binge Alcohol during Adolescence Induce Long-Term Effects on Anxiety-like Behavior and the Expression of the Endocannabinoid System in Male Rats.Biomedicines. 2022 Mar 3;10(3):593. doi: 10.3390/biomedicines10030593. Biomedicines. 2022. PMID: 35327395 Free PMC article.
-
Cross-talk between the HPA axis and addiction-related regions in stressful situations.Heliyon. 2023 Apr 17;9(4):e15525. doi: 10.1016/j.heliyon.2023.e15525. eCollection 2023 Apr. Heliyon. 2023. PMID: 37151697 Free PMC article. Review.
-
A common genetic variant in fatty acid amide hydrolase is linked to alterations in fear extinction neural circuitry in a racially diverse, nonclinical sample of adults.J Neurosci Res. 2022 Mar;100(3):744-761. doi: 10.1002/jnr.24860. Epub 2021 May 29. J Neurosci Res. 2022. PMID: 34051704 Free PMC article.
-
Comorbidity of Post-Traumatic Stress Disorder and Alcohol Use Disorder: Animal Models and Associated Neurocircuitry.Int J Mol Sci. 2022 Dec 26;24(1):388. doi: 10.3390/ijms24010388. Int J Mol Sci. 2022. PMID: 36613829 Free PMC article. Review.
References
-
- Roberts A.L., Gilman S.E., Breslau J., Breslau N., Koenen K.C. Race/Ethnic Differences in Exposure to Traumatic Events, Development of Post-Traumatic Stress Disorder, and Treatment-Seeking for Post-Traumatic Stress Disorder in the United States. Psychol. Med. 2011;41:71–83. doi: 10.1017/S0033291710000401. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

