Cancer-Associated Adipocytes in Breast Cancer: Causes and Consequences
- PMID: 33917351
- PMCID: PMC8038661
- DOI: 10.3390/ijms22073775
Cancer-Associated Adipocytes in Breast Cancer: Causes and Consequences
Abstract
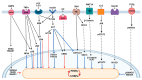
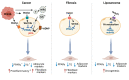
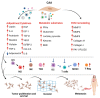
Breast cancer progression is highly dependent on the heterotypic interaction between tumor cells and stromal cells of the tumor microenvironment. Cancer-associated adipocytes (CAAs) are emerging as breast cancer cell partners favoring proliferation, invasion, and metastasis. This article discussed the intersection between extracellular signals and the transcriptional cascade that regulates adipocyte differentiation in order to appreciate the molecular pathways that have been described to drive adipocyte dedifferentiation. Moreover, recent studies on the mechanisms through which CAAs affect the progression of breast cancer were reviewed, including adipokine regulation, metabolic reprogramming, extracellular matrix remodeling, and immune cell modulation. An in-depth understanding of the complex vicious cycle between CAAs and breast cancer cells is crucial for designing novel strategies for new therapeutic interventions.
Keywords: adipocyte dedifferentiation; adipogenesis; breast cancer; cancer-associated adipocytes (CAA); signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

