Benzoquinoline Derivatives: A Straightforward and Efficient Route to Antibacterial and Antifungal Agents
- PMID: 33917439
- PMCID: PMC8067460
- DOI: 10.3390/ph14040335
Benzoquinoline Derivatives: A Straightforward and Efficient Route to Antibacterial and Antifungal Agents
Abstract
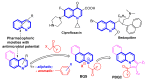
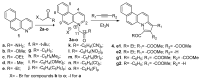
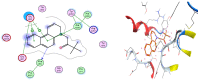
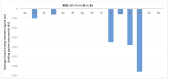
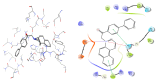
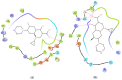
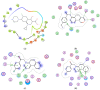
We report here the design, synthesis, experimental and in silico evaluation of the antibacterial and antifungal activity of some new benzo[f]quinoline derivatives. Two classes of benzo[f]quinolinium derivatives-(benzo[f]quinolinium salts (BQS) and pyrrolobenzo[f]quinolinium cycloadducts (PBQC)-were designed and obtained in two steps via a direct and facile procedure: quaternization followed by a cycloaddition reaction. The synthesized compounds were characterized by elemental and spectral analysis (FT-IR, 1H-NMR, 13C-NMR). The antimicrobial assay reveals that the BQS salts have an excellent quasi-nonselective antifungal activity against the fungus Candida albicans (some of them higher that the control drug nystatin) and very good antibacterial activity against the Gram positive bacterium Staphylococcus aureus. The PBQC compounds are inactive. Analysis of the biological data reveals interesting SAR correlations in the benzo[f]quinolinium series of compounds. The in silico studies furnished important data concerning the pharmacodynamics, pharmacokinetics and ADMET parameters of the BQS salts. Studies of the interaction of each BQS salt 3a-o with ATP synthase in the formed complex, reveal that salts 3j, 3i, and 3n have the best fit in a complex with ATP synthase. Study of the interaction of each BQS salt 3a-o with TOPO II in the formed complex reveals that salts 3j and 3n have the best-fit in complex with TOPO II. The in silico ADMET studies reveal that the BQS salts have excellent drug-like properties, including a low toxicity profile. Overall, the experimental and in silico studies indicate that compounds 3e and 3f (from the aliphatic series), respectively, and 3i, 3j and 3n (from the aromatic series), are promising leading drug candidates.
Keywords: ADMET; SAR/QSAR; antibacterial; antifungal; benzo[f]quinoline; cycloadducts; mechanism of action; molecular docking; salts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
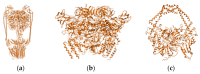
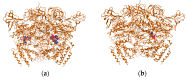
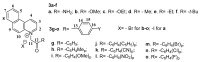
References
-
- WHO Global Strategy for Containment of Antimicrobial Resistance. [(accessed on 24 February 2021)]; Available online: https://www.who.int/drugresistance/WHO_Global_Strategy_English.pdf.
-
- Silverman R.B., Holladay M.W. The Organic Chemistry of DRUG design and Drug Action. 3rd ed. Academic Press; London, UK: 2014.
-
- Brunton L., Knollmann B., Hilal-Dandan R. Goodman & Gilman’s the Pharmacological Basis of Therapeutics. 13th ed. McGraw-Hill; New York, NY, USA: 2013.
-
- Eicher T., Hauptmann S., Speicher A. The Chemistry of Heterocycles: Structures, Reactions, Synthesis, and Applications. 3rd ed. Wiley-VCH; Weinheim, Germany: 2013.
-
- Kumari L.S., Mazumder A., Kumar V., Gupta S. Synthesis and biological potentials of quinoline analogues: A review of literature. Mini-Rev. Org. Chem. 2019;16:653–688. doi: 10.2174/1570193X16666190213105146. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

