Drugs That Induce or Cause Deterioration of Myasthenia Gravis: An Update
- PMID: 33917535
- PMCID: PMC8038781
- DOI: 10.3390/jcm10071537
Drugs That Induce or Cause Deterioration of Myasthenia Gravis: An Update
Abstract
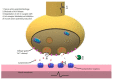
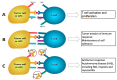
Myasthenia gravis (MG) is an autoimmune neuromuscular disorder which is characterized by presence of antibodies against acetylcholine receptors (AChRs) or other proteins of the postsynaptic membrane resulting in damage to postsynaptic membrane, decreased number of AChRs or blocking of the receptors by autoantibodies. A number of drugs such as immune checkpoint inhibitors, penicillamine, tyrosine kinase inhibitors and interferons may induce de novo MG by altering the immune homeostasis mechanisms which prevent emergence of autoimmune diseases such as MG. Other drugs, especially certain antibiotics, antiarrhythmics, anesthetics and neuromuscular blockers, have deleterious effects on neuromuscular transmission, resulting in increased weakness in MG or MG-like symptoms in patients who do not have MG, with the latter usually being under medical circumstances such as kidney failure. This review summarizes the drugs which can cause de novo MG, MG exacerbation or MG-like symptoms in nonmyasthenic patients.
Keywords: aminoglycoside; anesthesia; antibiotics; checkpoint inhibitor; corticosteroid; fluoroquinolone; macrolide; myasthenia gravis; sugammadex; tyrosine kinase inhibitor.
Conflict of interest statement
Rezania has received honoraria from Akcea, Alnylam, Alexion, Grifols, and Kabafusion for serving in advisory meetings, speaker, or as a consultant. Soliven, Sheikh and Alvi have nothing to disclose.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

