Excitatory Effects of Calcitonin Gene-Related Peptide (CGRP) on Superficial Sp5C Neurons in Mouse Medullary Slices
- PMID: 33917574
- PMCID: PMC8038766
- DOI: 10.3390/ijms22073794
Excitatory Effects of Calcitonin Gene-Related Peptide (CGRP) on Superficial Sp5C Neurons in Mouse Medullary Slices
Abstract
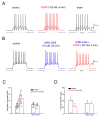
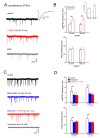
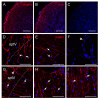
The neuromodulator calcitonin gene-related peptide (CGRP) is known to facilitate nociceptive transmission in the superficial laminae of the spinal trigeminal nucleus caudalis (Sp5C). The central effects of CGRP in the Sp5C are very likely to contribute to the activation of central nociceptive pathways leading to attacks of severe headaches like migraine. To examine the potential impacts of CGRP on laminae I/II neurons at cellular and synaptic levels, we performed whole-cell patch-clamp recordings in juvenile mouse brainstem slices. First, we tested the effect of CGRP on cell excitability, focusing on neurons with tonically firing action potentials upon depolarizing current injection. CGRP (100 nM) enhanced tonic discharges together with membrane depolarization, an excitatory effect that was significantly reduced when the fast synaptic transmissions were pharmacologically blocked. However, CGRP at 500 nM was capable of exciting the functionally isolated cells, in a nifedipine-sensitive manner, indicating its direct effect on membrane intrinsic properties. In voltage-clamped cells, 100 nM CGRP effectively increased the frequency of excitatory synaptic inputs, suggesting its preferential presynaptic effect. Both CGRP-induced changes in cell excitability and synaptic drives were prevented by the CGRP receptor inhibitor BIBN 4096BS. Our data provide evidence that CGRP increases neuronal activity in Sp5C superficial laminae by dose-dependently promoting excitatory synaptic drive and directly enhancing cell intrinsic properties. We propose that the combination of such pre- and postsynaptic actions of CGRP might underlie its facilitation in nociceptive transmission in situations like migraine with elevated CGRP levels.
Keywords: calcitonin gene-related peptide; cell excitability; excitatory postsynaptic currents; migraine; spinal trigeminal nucleus caudalis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Neuropeptide effects in the trigeminal system: pathophysiology and clinical relevance in migraine.Keio J Med. 2011;60(3):82-9. doi: 10.2302/kjm.60.82. Keio J Med. 2011. PMID: 21979827 Review.
-
Calcitonin gene-related peptide (CGRP) and its receptor components in human and rat spinal trigeminal nucleus and spinal cord at C1-level.BMC Neurosci. 2011 Nov 10;12:112. doi: 10.1186/1471-2202-12-112. BMC Neurosci. 2011. PMID: 22074408 Free PMC article.
-
Calcitonin gene-related peptide (CGRP) exerts membrane, cellular and synaptic actions on serotonergic dorsal raphe neurons ex vivo: Functional implications for a role in dorsal raphe-controlled functions.Neuropharmacology. 2025 Aug 1;273:110457. doi: 10.1016/j.neuropharm.2025.110457. Epub 2025 Apr 4. Neuropharmacology. 2025. PMID: 40189018
-
Pain-related synaptic plasticity in spinal dorsal horn neurons: role of CGRP.Mol Pain. 2006 Sep 26;2:31. doi: 10.1186/1744-8069-2-31. Mol Pain. 2006. PMID: 17002803 Free PMC article.
-
CGRP receptor antagonism and migraine therapy.Curr Protein Pept Sci. 2013 Aug;14(5):386-92. doi: 10.2174/13892037113149990055. Curr Protein Pept Sci. 2013. PMID: 23745702 Review.
Cited by
-
Serum CGRP in migraine patients using erenumab as preventive treatment.J Headache Pain. 2022 Sep 12;23(1):120. doi: 10.1186/s10194-022-01483-z. J Headache Pain. 2022. PMID: 36089587 Free PMC article.
-
Transcutaneous occipital nerve stimulation alleviated migraine related pain by regulating synaptic plasticity and CGRP expression in the periaqueductal gray of male rats.J Headache Pain. 2025 Mar 28;26(1):61. doi: 10.1186/s10194-025-02006-2. J Headache Pain. 2025. PMID: 40155829 Free PMC article.
-
Suppression of mechanical hypersensitivity and change in the expression of the dopamine D2 receptor by administration of anti-CGRP antibody into the trigeminal ganglion in trigeminal neuropathic pain model rats.PLoS One. 2025 May 14;20(5):e0323810. doi: 10.1371/journal.pone.0323810. eCollection 2025. PLoS One. 2025. PMID: 40367052 Free PMC article.
-
CGRP physiology, pharmacology, and therapeutic targets: migraine and beyond.Physiol Rev. 2023 Apr 1;103(2):1565-1644. doi: 10.1152/physrev.00059.2021. Epub 2022 Dec 1. Physiol Rev. 2023. PMID: 36454715 Free PMC article. Review.
-
The Anti-CGRP Antibody Fremanezumab Lowers CGRP Release from Rat Dura Mater and Meningeal Blood Flow.Cells. 2022 May 28;11(11):1768. doi: 10.3390/cells11111768. Cells. 2022. PMID: 35681463 Free PMC article.
References
-
- Lennerz J.K., Rühle V., Ceppa E.P., Neuhuber W.L., Bunnett N.W., Grady E.F., Messlinger K. Calcitonin Receptor-like Receptor (CLR), Receptor Activity-Modifying Protein 1 (RAMP1), and Calcitonin Gene-Related Peptide (CGRP) Immunoreactivity in the Rat Trigeminovascular System: Differences between Peripheral and Central CGRP Receptor Distribution. J. Comp. Neurol. 2008;507:1277–1299. doi: 10.1002/cne.21607. - DOI - PubMed
-
- Ray B.S., Wolff H.G. Experimental Studies on Headache: Pain Sensitive Structures of the Head and Their Significance in Headache. Arch. Surg. 1940;1:813–856. doi: 10.1001/archsurg.1940.01210040002001. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

