The Role of Hydrogen Bonding in Paracetamol-Solvent and Paracetamol-Hydrogel Matrix Interactions
- PMID: 33917724
- PMCID: PMC8068172
- DOI: 10.3390/ma14081842
The Role of Hydrogen Bonding in Paracetamol-Solvent and Paracetamol-Hydrogel Matrix Interactions
Abstract
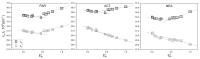
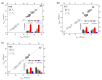
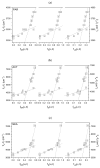
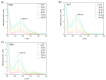
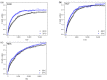
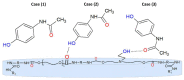
The photophysical and photochemical properties of antipyretic drug - paracetamol (PAR) and its two analogs with different substituents (acetanilide (ACT) and N-ethylaniline (NEA)) in 14 solvents of different polarity were investigated by the use of steady-state spectroscopic technique and quantum-chemical calculations. As expected, the results show that the spectroscopic behavior of PAR, ACT, and NEA is highly dependent on the nature of the solute-solvent interactions (non-specific (dipole-dipole) and specific (hydrogen bonding)). To characterize these interactions, the multiparameter regression analysis proposed by Catalán was used. In order to obtain a deeper insight into the electronic and optical properties of the studied molecules, the difference of the dipole moments of a molecule in the ground and excited state were determined using the theory proposed by Lippert, Mataga, McRae, Bakhshiev, Bilot, and Kawski. Additionally, the influence of the solute polarizability on the determined dipole moments was discussed. The results of the solvatochromic studies were related to the observations of the release kinetics of PAR, ACT, and NEA from polyurethane hydrogels. The release kinetics was analyzed using the Korsmayer-Peppas and Hopfenberg models. Finally, the influence of the functional groups of the investigated compounds on the release time from the hydrogel matrix was analyzed.
Keywords: drug release; hydrogel matrix; hydrogen bond; paracetamol; solvatochromism; solvent effects.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Tekade R. The Future of Pharmaceutical Product Development and Research. Academic Press; Cambridge, MA, USA: 2020.
-
- Saboo S., Kestur U.S., Flaherty D.P., Taylor L.S. Congruent Release of Drug and Polymer from Amorphous Solid Dispersions: Insights into the Role of Drug-Polymer Hydrogen Bonding, Surface Crystallization, and Glass Transition. Mol. Pharm. 2020;17:1261. doi: 10.1021/acs.molpharmaceut.9b01272. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

