Mitigating Future Respiratory Virus Pandemics: New Threats and Approaches to Consider
- PMID: 33917745
- PMCID: PMC8068197
- DOI: 10.3390/v13040637
Mitigating Future Respiratory Virus Pandemics: New Threats and Approaches to Consider
Abstract
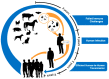
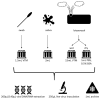
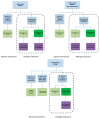
Despite many recent efforts to predict and control emerging infectious disease threats to humans, we failed to anticipate the zoonotic viruses which led to pandemics in 2009 and 2020. The morbidity, mortality, and economic costs of these pandemics have been staggering. We desperately need a more targeted, cost-efficient, and sustainable strategy to detect and mitigate future zoonotic respiratory virus threats. Evidence suggests that the transition from an animal virus to a human pathogen is incremental and requires a considerable number of spillover events and considerable time before a pandemic variant emerges. This evolutionary view argues for the refocusing of public health resources on novel respiratory virus surveillance at human-animal interfaces in geographical hotspots for emerging infectious diseases. Where human-animal interface surveillance is not possible, a secondary high-yield, cost-efficient strategy is to conduct novel respiratory virus surveillance among pneumonia patients in these same hotspots. When novel pathogens are discovered, they must be quickly assessed for their human risk and, if indicated, mitigation strategies initiated. In this review, we discuss the most common respiratory virus threats, current efforts at early emerging pathogen detection, and propose and defend new molecular pathogen discovery strategies with the goal of preempting future pandemics.
Keywords: emerging viruses; molecular detection; pathogen discovery; respiratory viruses.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Centers for Disease Control and Prevention SARS Basics Fact Sheet. [(accessed on 26 February 2021)]; Available online: https://www.cdc.gov/sars/about/fs-sars.html.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

