Differential Therapeutic Effect of Extracellular Vesicles Derived by Bone Marrow and Adipose Mesenchymal Stem Cells on Wound Healing of Diabetic Ulcers and Correlation to Their Cargoes
- PMID: 33917759
- PMCID: PMC8068154
- DOI: 10.3390/ijms22083851
Differential Therapeutic Effect of Extracellular Vesicles Derived by Bone Marrow and Adipose Mesenchymal Stem Cells on Wound Healing of Diabetic Ulcers and Correlation to Their Cargoes
Abstract
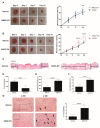
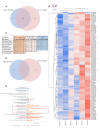
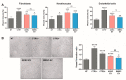
Extracellular vesicles (EVs) derived from mesenchymal stem cells isolated from both bone marrow (BMSCs) and adipose tissue (ADSCs) show potential therapeutic effects. These vesicles often show a similar beneficial effect on tissue regeneration, but in some contexts, they exert different biological properties. To date, a comparison of their molecular cargo that could explain the different biological effect is not available. Here, we demonstrated that ADSC-EVs, and not BMSC-EVs, promote wound healing on a murine model of diabetic wounds. Besides a general similarity, the bioinformatic analysis of their protein and miRNA cargo highlighted important differences between these two types of EVs. Molecules present exclusively in ADSC-EVs were highly correlated to angiogenesis, whereas those expressed in BMSC-EVs were preferentially involved in cellular proliferation. Finally, in vitro analysis confirmed that both ADSC and BMSC-EVs exploited beneficial effect on cells involved in skin wound healing such as fibroblasts, keratinocytes and endothelial cells, but through different cellular processes. Consistent with the bioinformatic analyses, BMSC-EVs were shown to mainly promote proliferation, whereas ADSC-EVs demonstrated a major effect on angiogenesis. Taken together, these results provide deeper comparative information on the cargo of ADSC-EVs and BMSC-EVs and the impact on regenerative processes essential for diabetic wound healing.
Keywords: MSC; adipose; bone marrow; cargo; diabetes; exosome; extracellular vesicle; mesenchymal; therapy; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., et al. Minimal information for studies of Extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

