Novel Ring Systems: Spiro[Cycloalkane] Derivatives of Triazolo- and Tetrazolo-Pyridazines
- PMID: 33917797
- PMCID: PMC8068119
- DOI: 10.3390/molecules26082140
Novel Ring Systems: Spiro[Cycloalkane] Derivatives of Triazolo- and Tetrazolo-Pyridazines
Abstract
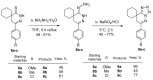
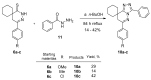
In orderto synthesize new pyridazine derivatives anellated with different nitrogen heterocyclic moieties, spiro[cycloalkane]pyridazinones were transformed into the corresponding thioxo derivatives via a reaction with phosphorus pentasulfide. The reaction of the formed 2,3-diazaspiro[5.5] undec-3-ene-1-thiones with hydrazine provided the corresponding 1-hydrazono-2,3-diazaspiro[5.5] undec-3-ene, whose diazotization led to the desired spiro[cyclohexane-1,8'-tetrazolo[1,5-b]pyridazines. The reaction of dihydropyridazinethiones with benzhydrazide afforded the corresponding 7H-spiro[[1,2,4]triazolo[4,3-b]pyridazin-8,1'-cyclohexanes]. As a result of our work, seven new pyridazinethione intermediates were prepared, which served as starting materials for the synthesis of two kinds of new ring systems: tetrazolo-pyridazines and triazolo-pyridazines. The six new annulated derivatives were characterized by physicochemical parameters. The new N-heterocycles are valuable members of the large family of pyridazines.
Keywords: N-heterocycles; diazotization; pyridazine derivatives; tetrazolo-pyridazines; thionation; triazolo-pyridazines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
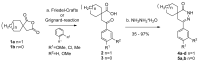
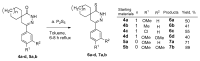

Similar articles
-
A New Synthesis of Poly Heterocyclic Compounds Containing [1,2,4]triazolo and [1,2,3,4]tetrazolo Moieties and their DFT Study as Expected Anti-cancer Reagents.Curr Org Synth. 2020;17(3):211-223. doi: 10.2174/1570179417666200226092516. Curr Org Synth. 2020. PMID: 32101129
-
Pyridazine derivatives and related compounds, part 9. tetrazolo[1,5-b]pyridazine-8-carbohydrazide: synthesis and some reactions.Boll Chim Farm. 2002 Mar-Apr;141(2):118-21. Boll Chim Farm. 2002. PMID: 12135159
-
Syntheses and Applications of 1,2,3-Triazole-Fused Pyrazines and Pyridazines.Molecules. 2022 Jul 22;27(15):4681. doi: 10.3390/molecules27154681. Molecules. 2022. PMID: 35897857 Free PMC article. Review.
-
Synthesis and pharmacological evaluation of 6,8-diaryl 1,2,4-triazolo [4,3,-b] and 1,2,3,4,-tetrazolo [1,5-b] pyridazines.Farmaco. 1990 Mar;45(3):331-40. doi: 10.1002/chin.199048183. Farmaco. 1990. PMID: 1974435
-
Some recent approaches of biologically active substituted pyridazine and phthalazine drugs.Curr Med Chem. 2012;19(18):2984-91. doi: 10.2174/092986712800672139. Curr Med Chem. 2012. PMID: 22519394 Review.
Cited by
-
Novel Pyridothienopyrimidine Derivatives: Design, Synthesis and Biological Evaluation as Antimicrobial and Anticancer Agents.Molecules. 2022 Jan 26;27(3):803. doi: 10.3390/molecules27030803. Molecules. 2022. PMID: 35164067 Free PMC article.
References
-
- Wermuth C.G. Are pyridazines privileged structures? Med. Chem. Comm. 2011;2:935–941. doi: 10.1039/C1MD00074H. - DOI
-
- Castle R.N., editor. The Chemistry of Heterocyclic Compounds Pyridazines. Volume 28. Wiley; Hoboken, NJ, USA: 2009.
-
- Heinisch G., Holzer W., Kunz F., Langer T., Lukavsky P., Pechlaner C., Weissenberger H. On the Bioisosteric Potential of Diazines: Diazine Analogues of the Combined Thromboxane A2 Receptor Antagonist and Synthetase Inhibitor Ridogrel. J. Med. Chem. 1996;39:4058–4064. doi: 10.1021/jm960341g. - DOI - PubMed
-
- Asif M. The Pharmacological Importance of Some Diazine Containing Drug Molecules. Sci. Online Pub. Trans. Org. Chem. 2014;1:1–17.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

