Dabrafenib Promotes Schwann Cell Differentiation by Inhibition of the MEK-ERK Pathway
- PMID: 33917810
- PMCID: PMC8068149
- DOI: 10.3390/molecules26082141
Dabrafenib Promotes Schwann Cell Differentiation by Inhibition of the MEK-ERK Pathway
Abstract
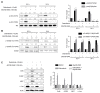
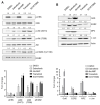
Schwann cell differentiation involves a dynamic interaction of signaling cascades. However, much remains to be elucidated regarding the function of signaling molecules that differ depending on the context in which the molecules are engaged. Here, we identified a small molecule, dabrafenib, which promotes Schwann cell differentiation in vitro and exploited this compound as a pharmacological tool to understand the molecular mechanisms regulating Schwann cell differentiation. The results indicated that dabrafenib inhibited ERK phosphorylation and enhanced ErbB2 autophosphorylation and Akt phosphorylation, and the effects of dabrafenib on ErbB2 and Akt phosphorylation were phenocopied by pharmacological inhibition of the MEK-ERK signaling pathway. However, the small molecule inhibitors of MEK and ERK had no effect on the expression of Oct6 and EGR2, which are key transcription factors that drive Schwann cell differentiation. In addition, pharmacological inhibition of phosphatidylinositol-3-kinase (PI3K) almost completely interfered with dabrafenib-induced Schwann cell differentiation. These results suggest that the ErbB2-PI3K-Akt axis is required for the induction of Schwann cell differentiation by dabrafenib in vitro. Although additional molecules targeted by dabrafenib remain to be identified, our data provides insights into the crosstalk that exists between the MEK-ERK signaling pathway and the PI3K-Akt axis in Schwann cell differentiation.
Keywords: ERK; ErbB2; Schwann cell; dabrafenib; differentiation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Carboxymethylated chitosan stimulates proliferation of Schwann cells in vitro via the activation of the ERK and Akt signaling pathways.Eur J Pharmacol. 2011 Sep 30;667(1-3):195-201. doi: 10.1016/j.ejphar.2011.06.001. Epub 2011 Jun 16. Eur J Pharmacol. 2011. PMID: 21699895
-
Quantifying ERK activity in response to inhibition of the BRAFV600E-MEK-ERK cascade using mathematical modelling.Br J Cancer. 2021 Nov;125(11):1552-1560. doi: 10.1038/s41416-021-01565-w. Epub 2021 Oct 7. Br J Cancer. 2021. PMID: 34621046 Free PMC article.
-
Opposing extracellular signal-regulated kinase and Akt pathways control Schwann cell myelination.J Neurosci. 2004 Jul 28;24(30):6724-32. doi: 10.1523/JNEUROSCI.5520-03.2004. J Neurosci. 2004. PMID: 15282275 Free PMC article.
-
Neuregulin signaling through a PI3K/Akt/Bad pathway in Schwann cell survival.Mol Cell Neurosci. 2001 Apr;17(4):761-7. doi: 10.1006/mcne.2000.0967. Mol Cell Neurosci. 2001. PMID: 11312610
-
Signaling axis in schwann cell proliferation and differentiation.Mol Neurobiol. 2006 Feb;33(1):51-62. doi: 10.1385/mn:33:1:051. Mol Neurobiol. 2006. PMID: 16388110 Review.
Cited by
-
Roles of ERK signaling pathway in regulating myelination of the peripheral nervous system.Front Mol Neurosci. 2025 Jun 13;18:1617976. doi: 10.3389/fnmol.2025.1617976. eCollection 2025. Front Mol Neurosci. 2025. PMID: 40584403 Free PMC article. Review.
-
Neuron-Schwann cell interactions in peripheral nervous system homeostasis, disease, and preclinical treatment.Front Cell Neurosci. 2023 Oct 12;17:1248922. doi: 10.3389/fncel.2023.1248922. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37900588 Free PMC article. Review.
-
Curcuma phaeocaulis Inhibits NLRP3 Inflammasome in Macrophages and Ameliorates Nanoparticle-Induced Airway Inflammation in Mice.Molecules. 2022 Mar 24;27(7):2101. doi: 10.3390/molecules27072101. Molecules. 2022. PMID: 35408502 Free PMC article.
-
Bioinformatics analysis combined with experimental validation reveals the novel mechanisms of multi-targets of dapagliflozin attenuating diabetic liver injury.Front Endocrinol (Lausanne). 2025 May 12;16:1519153. doi: 10.3389/fendo.2025.1519153. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40438398 Free PMC article.
-
Platelet-Rich Plasma Gel-Loaded Collagen/Chitosan Composite Film Accelerated Rat Sciatic Nerve Injury Repair.ACS Omega. 2023 Jan 11;8(3):2931-2941. doi: 10.1021/acsomega.2c05351. eCollection 2023 Jan 24. ACS Omega. 2023. PMID: 36713745 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

