Melting Curve Analysis of Aptachains: Adenosine Detection with Internal Calibration
- PMID: 33917864
- PMCID: PMC8068264
- DOI: 10.3390/bios11040112
Melting Curve Analysis of Aptachains: Adenosine Detection with Internal Calibration
Abstract
Small molecules are ubiquitous in nature and their detection is relevant in various domains. However, due to their size, sensitive and selective probes are difficult to select and the detection methods are generally indirect. In this study, we introduced the use of melting curve analysis of aptachains based on split-aptamers for the detection of adenosine. Aptamers, short oligonucleotides, are known to be particularly efficient probes compared to antibodies thanks to their advantageous probe/target size ratio. Aptachains are formed from dimers with dangling ends followed by the split-aptamer binding triggered by the presence of the target. The high melting temperature of the dimers served as a calibration for the detection/quantification of the target based on the height and/or temperature shift of the aptachain melting peak.
Keywords: aptachain self-assembly; calibration/normalization; melting temperature; small molecule detection; split-aptamers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





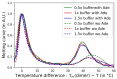

References
-
- Qi X., Yan X., Zhao Y., Li L., Wang S. Highly Sensitive and Specific Detection of Small Molecules Using Advanced Aptasensors Based on Split Aptamers: A Review. Trac. Trends Anal. Chem. 2020;133:116069. doi: 10.1016/j.trac.2020.116069. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

