Synthesis, Antiprotozoal Activity, and Cheminformatic Analysis of 2-Phenyl-2 H-Indazole Derivatives
- PMID: 33917871
- PMCID: PMC8068258
- DOI: 10.3390/molecules26082145
Synthesis, Antiprotozoal Activity, and Cheminformatic Analysis of 2-Phenyl-2 H-Indazole Derivatives
Abstract
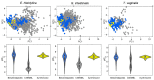
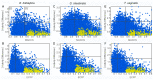
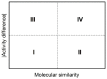
Indazole is an important scaffold in medicinal chemistry. At present, the progress on synthetic methodologies has allowed the preparation of several new indazole derivatives with interesting pharmacological properties. Particularly, the antiprotozoal activity of indazole derivatives have been recently reported. Herein, a series of 22 indazole derivatives was synthesized and studied as antiprotozoals. The 2-phenyl-2H-indazole scaffold was accessed by a one-pot procedure, which includes a combination of ultrasound synthesis under neat conditions as well as Cadogan's cyclization. Moreover, some compounds were derivatized to have an appropriate set to provide structure-activity relationships (SAR) information. Whereas the antiprotozoal activity of six of these compounds against E. histolytica, G. intestinalis, and T. vaginalis had been previously reported, the activity of the additional 16 compounds was evaluated against these same protozoa. The biological assays revealed structural features that favor the antiprotozoal activity against the three protozoans tested, e.g., electron withdrawing groups at the 2-phenyl ring. It is important to mention that the indazole derivatives possess strong antiprotozoal activity and are also characterized by a continuous SAR.
Keywords: Entamoeba histolytica; Giardia intestinalis; Trichomonas vaginalis; indazole; structure-activity relationships.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Synthesis and Biological Evaluation of 2H-Indazole Derivatives: Towards Antimicrobial and Anti-Inflammatory Dual Agents.Molecules. 2017 Oct 31;22(11):1864. doi: 10.3390/molecules22111864. Molecules. 2017. PMID: 29088121 Free PMC article.
-
Synthesis, antiprotozoal activity, and chemoinformatic analysis of 2-(methylthio)-1H-benzimidazole-5-carboxamide derivatives: Identification of new selective giardicidal and trichomonicidal compounds.Eur J Med Chem. 2017 Sep 8;137:211-220. doi: 10.1016/j.ejmech.2017.05.058. Epub 2017 May 29. Eur J Med Chem. 2017. PMID: 28595066
-
Expanding the antiprotozoal activity and the mechanism of action of n-butyl and iso-butyl ester of quinoxaline-1,4-di-N-oxide derivatives against Giardia lamblia, Trichomonas vaginalis, and Entamoeba histolytica. An in vitro and in silico approach.J Enzyme Inhib Med Chem. 2024 Dec;39(1):2413018. doi: 10.1080/14756366.2024.2413018. Epub 2024 Oct 29. J Enzyme Inhib Med Chem. 2024. PMID: 39470324 Free PMC article.
-
Drug targets and mechanisms of resistance in the anaerobic protozoa.Clin Microbiol Rev. 2001 Jan;14(1):150-64. doi: 10.1128/CMR.14.1.150-164.2001. Clin Microbiol Rev. 2001. PMID: 11148007 Free PMC article. Review.
-
Recent Advances in Indazole-Containing Derivatives: Synthesis and Biological Perspectives.Molecules. 2018 Oct 26;23(11):2783. doi: 10.3390/molecules23112783. Molecules. 2018. PMID: 30373212 Free PMC article. Review.
Cited by
-
Ultrasound-Assisted Synthesis of Fluorescent Azatetracyclic Derivatives: An Energy-Efficient Approach.Molecules. 2022 May 16;27(10):3180. doi: 10.3390/molecules27103180. Molecules. 2022. PMID: 35630657 Free PMC article.
-
Fluorescent Azasteroids through Ultrasound Assisted Cycloaddition Reactions.Molecules. 2021 Aug 23;26(16):5098. doi: 10.3390/molecules26165098. Molecules. 2021. PMID: 34443688 Free PMC article.
-
Recent Advances in Synthetic Strategies and Biological Properties of Indazole Scaffolds: A Review.Top Curr Chem (Cham). 2025 Jul 1;383(3):26. doi: 10.1007/s41061-025-00509-9. Top Curr Chem (Cham). 2025. PMID: 40591193 Free PMC article. Review.
-
Heterocycles in Medicinal Chemistry II.Molecules. 2024 Oct 11;29(20):4810. doi: 10.3390/molecules29204810. Molecules. 2024. PMID: 39459179 Free PMC article.
-
An Overview of Mucosa-Associated Protozoa: Challenges in Chemotherapy and Future Perspectives.Front Cell Infect Microbiol. 2022 Apr 25;12:860442. doi: 10.3389/fcimb.2022.860442. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35548465 Free PMC article. Review.
References
-
- Thangadurai A., Minu M., Wakode S., Agrawal S., Narasimhan B. Indazole: A medicinally important heterocyclic moiety. Med. Chem. Res. 2012;21:1509–1523. doi: 10.1007/s00044-011-9631-3. - DOI
-
- Hugo C., Alejandra G., Mercedes G., Vicente J.A., Carmen O.d.O. Pharmacological properties of indazole derivatives: Recent developments. Mini. Rev. Med. Chem. 2005;5:869–878. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

