Chemical Hemisynthesis of Sulfated Cyanidin-3- O-Glucoside and Cyanidin Metabolites
- PMID: 33917913
- PMCID: PMC8068276
- DOI: 10.3390/molecules26082146
Chemical Hemisynthesis of Sulfated Cyanidin-3- O-Glucoside and Cyanidin Metabolites
Abstract
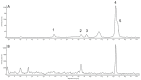
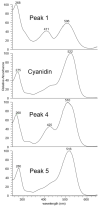
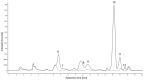
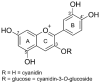
The metabolism of anthocyanins in humans is still not fully understood, which is partly due to the lack of reference compounds. It is known that sulfation is one way of the complex phase II biotransformation mechanism. Therefore, cyanidin-3-O-glucoside and the cyanidin aglycone were chemically converted to their sulfates by reaction with sulfur trioxide-N-triethylamine complex in dimethylformamide. The reaction products were characterized by UHPLC coupled to linear ion trap and IMS-QTOF mass spectrometry. Based on MS data, retention times, and UV-Vis spectra, the compounds could tentatively be assigned to A-, C-, or B-ring sulfates. Analysis of urine samples from two volunteers after ingestion of commercial blackberry nectar demonstrated the presence of two sulfated derivatives of the cyanidin aglycone and one sulfated derivative of the cyanidin-3-O-glucoside. It was found that both the A ring and the B ring are sulfated by human enzymes. This study marks an important step toward a better understanding of anthocyanin metabolism.
Keywords: HRMS; LC-IMS; anthocyanins; cyanidin; cyanidin-3-O-glucoside; metabolites; sulfation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Horbowicz M., Kosson R., Grzesiuk A., Dębski H. Anthocyanins of Fruits and Vegetables—Their Occurrence, Analysis and Role in Human Nutrition. Veg. Crop. Res. Bull. 2008;68:5–22. doi: 10.2478/v10032-008-0001-8. - DOI
-
- Fossen T., Cabrita L., Andersen O.M. Colour and stability of pure anthocyanins influenced by pH including the alkaline region. Food Chem. 1998;63:435–440. doi: 10.1016/S0308-8146(98)00065-X. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

