Protocatechuic Aldehyde Inhibits α-MSH-Induced Melanogenesis in B16F10 Melanoma Cells via PKA/CREB-Associated MITF Downregulation
- PMID: 33917915
- PMCID: PMC8068260
- DOI: 10.3390/ijms22083861
Protocatechuic Aldehyde Inhibits α-MSH-Induced Melanogenesis in B16F10 Melanoma Cells via PKA/CREB-Associated MITF Downregulation
Abstract
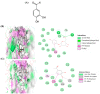
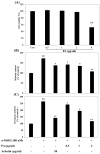
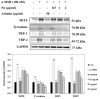
Protocatechuic aldehyde (PA) is a naturally occurring phenolic compound that is a potent inhibitor of mushroom tyrosinase. However, the molecular mechanisms of the anti-melanogenesis activity of PA have not yet been reported. The aim of the current study was to clarify the melanogenesis inhibitory effects of PA and its molecular mechanisms in murine melanoma cells (B16F10). We first predicted the 3D structure of tyrosinase and used a molecular docking algorithm to simulate binding between tyrosinase and PA. These molecular modeling studies calculated a binding energy of -527.42 kcal/mol and indicated that PA interacts with Cu400 and 401, Val283, and His263. Furthermore, PA significantly decreased α-MSH-induced intracellular tyrosinase activity and melanin content in a dose-dependent manner. PA also inhibited key melanogenic proteins such as tyrosinase, tyrosinase-related protein 1 (TRP-1), and TRP-2 in α-MSH-stimulated B16F10 cells. In addition, PA decreased MITF expression levels by inhibiting phosphorylation of cAMP response element-binding protein (CREB) and cAMP-dependent protein kinase A (PKA). These results demonstrate that PA can effectively suppress melanin synthesis in melanoma cells. Taken together, our results show that PA could serve as a potential inhibitor of melanogenesis, and hence could be explored as a possible skin-lightening agent.
Keywords: anti-melanogenesis activity; melanoma cells; molecular mechanisms; protocatechuic aldehyde.
Conflict of interest statement
The authors declare that there are no conflict of interest.
Figures

References
-
- Huang H.C., Liao C.C., Peng C.C., Lim J.M., Siao J.H., Wei C.M., Chen C.C., Wu C.S., Chang T.M. Dihydromyricetin from Ampelopsis grossedentata inhibits melanogenesis through downregulation of MAPK, PKA and PKC signaling pathways. Chem. Biol. Interact. 2016;258:166–174. doi: 10.1016/j.cbi.2016.08.023. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

