Macrophages and Autoantibodies in Demyelinating Diseases
- PMID: 33917929
- PMCID: PMC8068327
- DOI: 10.3390/cells10040844
Macrophages and Autoantibodies in Demyelinating Diseases
Abstract
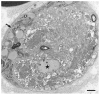
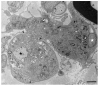
Myelin phagocytosis by macrophages has been an essential feature of demyelinating diseases in the central and peripheral nervous systems, including Guillain-Barré syndrome (GBS), chronic inflammatory demyelinating polyneuropathy (CIDP), and multiple sclerosis (MS). The discovery of autoantibodies, including anti-ganglioside GM1 antibodies in the axonal form of GBS, anti-neurofascin 155 and anti-contactin 1 antibodies in typical and distal forms of CIDP, and anti-aquaporin 4 antibodies in neuromyelitis optica, contributed to the understanding of the disease process in a subpopulation of patients conventionally diagnosed with demyelinating diseases. However, patients with these antibodies are now considered to have independent disease entities, including acute motor axonal neuropathy, nodopathy or paranodopathy, and neuromyelitis optica spectrum disorder, because primary lesions in these diseases are distinct from those in conventional demyelinating diseases. Therefore, the mechanisms underlying demyelination caused by macrophages remain unclear. Electron microscopy studies revealed that macrophages destroy myelin as if they are the principal players in the demyelination process. Recent studies suggest that macrophages seem to select specific sites of myelinated fibers, including the nodes of Ranvier, paranodes, and internodes, for the initiation of demyelination in individual cases, indicating that specific components localized to these sites play an important role in the behavior of macrophages that initiate myelin phagocytosis. Along with the search for autoantibodies, the ultrastructural characterization of myelin phagocytosis by macrophages is a crucial step in understanding the pathophysiology of demyelinating diseases and for the future development of targeted therapies.
Keywords: Schwann cell; demyelination; electron microscopy; macrophage; paranode; pathogenesis; pathology; the node of Ranvier; treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

