Degradation Products of Polychlorinated Biphenyls and Their In Vitro Transformation by Ligninolytic Fungi
- PMID: 33918084
- PMCID: PMC8070434
- DOI: 10.3390/toxics9040081
Degradation Products of Polychlorinated Biphenyls and Their In Vitro Transformation by Ligninolytic Fungi
Abstract
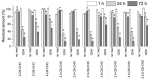
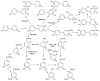
Metabolites of polychlorinated biphenyls (PCBs)-hydroxylated PCBs (OH-PCBs), chlorobenzyl alcohols (CB-OHs), and chlorobenzaldehydes (CB-CHOs)-were incubated in vitro with the extracellular liquid of Pleurotus ostreatus, which contains mainly laccase and low manganese-dependent peroxidase (MnP) activity. The enzymes were able to decrease the amount of most of the tested OH-PCBs by > 80% within 1 h; the removal of more recalcitrant OH-PCBs was greatly enhanced by the addition of the laccase mediator syringaldehyde. Conversely, glutathione substantially hindered the reaction, suggesting that it acted as a laccase inhibitor. Hydroxylated dibenzofuran and chlorobenzoic acid were identified as transformation products of OH-PCBs. The extracellular enzymes also oxidized the CB-OHs to the corresponding CB-CHOs on the order of hours to days; however, the mediated and nonmediated setups exhibited only slight differences, and the participating enzymes could not be determined. When CB-CHOs were used as the substrates, only partial transformation was observed. In an additional experiment, the extracellular liquid of Irpex lacteus, which contains predominantly MnP, was able to efficiently transform CB-CHOs with the aid of glutathione; mono- and di-chloroacetophenones were detected as transformation products. These results demonstrate that extracellular enzymes of ligninolytic fungi can act on a wide range of PCB metabolites, emphasizing their potential for bioremediation.
Keywords: Irpex lacteus; Pleurotus ostreatus; biodegradation; chlorobenzaldehydes; chlorobenzyl alcohols; hydroxylated PCBs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

