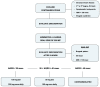
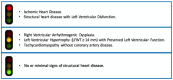
Flecainide How and When: A Practical Guide in Supraventricular Arrhythmias
- PMID: 33918105
- PMCID: PMC8036302
- DOI: 10.3390/jcm10071456
Flecainide How and When: A Practical Guide in Supraventricular Arrhythmias
Abstract
Transcatheter ablation was increasingly and successfully used to treat symptomatic drug refractory patients affected by supraventricular arrhythmias. Antiarrhythmic drug treatment still plays a major role in patient management, alone or combined with non-pharmacological therapies. Flecainide is an IC antiarrhythmic drug approved in 1984 from the Food and Drug Administration for the suppression of sustained ventricular tachycardia and later for acute cardioversion of atrial fibrillation and for sinus rhythm maintenance. Currently, flecainide is mostly used for sinus rhythm maintenance in atrial fibrillation (AF) patients without structural cardiomyopathy although recent studies enrolling different patient populations have demonstrated a good effectiveness and safety profile. How should we interpret the results of the CAST after the latest evidence? Is it possible to expand the indications of flecainide, and therefore, its use? This review aims to highlight the main characteristics of flecainide, as well as its optimal clinical use, delineating drug indications and contraindications and appropriate monitoring, based on the most recent evidence.
Keywords: CAST; atrial fibrillation; flecainide; flecainide controlled-release; supraventricular arrhythmias.
Conflict of interest statement
Lavalle, Santini, Forleo, Grimaldi, Badagliacca and Ricci have received speaker honoraria from Dompè SPA, Italy. L. Lanata is employee of Dompé SPA, Italy. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures
Similar articles
-
Flecainide in clinical practice.Cardiol J. 2023;30(3):473-482. doi: 10.5603/CJ.a2023.0018. Epub 2023 Mar 13. Cardiol J. 2023. PMID: 36908162 Free PMC article. Review.
-
Flecainide in Ventricular Arrhythmias: From Old Myths to New Perspectives.J Clin Med. 2021 Aug 20;10(16):3696. doi: 10.3390/jcm10163696. J Clin Med. 2021. PMID: 34441994 Free PMC article. Review.
-
Flecainide acetate for the treatment of atrial and ventricular arrhythmias.Expert Opin Pharmacother. 2013 Feb;14(3):347-57. doi: 10.1517/14656566.2013.759212. Epub 2013 Jan 7. Expert Opin Pharmacother. 2013. PMID: 23294160 Review.
-
Safety of flecainide.Drug Saf. 2012 Apr 1;35(4):273-89. doi: 10.2165/11599950-000000000-00000. Drug Saf. 2012. PMID: 22435343 Review.
-
Flecainide: Electrophysiological properties, clinical indications, and practical aspects.Pharmacol Res. 2019 Oct;148:104443. doi: 10.1016/j.phrs.2019.104443. Epub 2019 Sep 4. Pharmacol Res. 2019. PMID: 31493514 Review.
Cited by
-
Atrial Fibrillation: Rate Versus Rhythm Control.HCA Healthc J Med. 2023 Oct 30;4(5):329-339. doi: 10.36518/2689-0216.1564. eCollection 2023. HCA Healthc J Med. 2023. PMID: 37969851 Free PMC article. Review.
-
Flecainide in clinical practice.Cardiol J. 2023;30(3):473-482. doi: 10.5603/CJ.a2023.0018. Epub 2023 Mar 13. Cardiol J. 2023. PMID: 36908162 Free PMC article. Review.
-
A Rare Case Report of Flecainide-Induced Left Bundle Branch Block (LBBB) and Transient Cardiomyopathy.Cureus. 2023 Apr 5;15(4):e37184. doi: 10.7759/cureus.37184. eCollection 2023 Apr. Cureus. 2023. PMID: 37034143 Free PMC article.
-
Atrial fibrillation and sympatho-vagal imbalance: from the choice of the antiarrhythmic treatment to patients with syncope and ganglionated plexi ablation.Eur Heart J Suppl. 2023 Apr 26;25(Suppl C):C1-C6. doi: 10.1093/eurheartjsupp/suad075. eCollection 2023 May. Eur Heart J Suppl. 2023. PMID: 37125283 Free PMC article.
-
Is Low Heart Rate Variability Associated with Emotional Dysregulation, Psychopathological Dimensions, and Prefrontal Dysfunctions? An Integrative View.J Pers Med. 2021 Aug 31;11(9):872. doi: 10.3390/jpm11090872. J Pers Med. 2021. PMID: 34575648 Free PMC article. Review.
References
-
- Hindricks G., Potpara T., Dagres N., Arbelo E., Bax J.J., Blomström-Lundqvist C., Boriani G., Castella M., Dan G.-A., Dilaveris P.E., et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association of Cardio-Thoracic Surgery (EACTS) Eur. Heart J. 2020:ehaa612. doi: 10.1093/eurheartj/ehaa612. - DOI - PubMed
-
- Saksena S., Slee A., Waldo A.L., Freemantle N., Reynolds M., Rosenberg Y., Rathod S., Grant S., Thomas E., Wyse D.G. Cardiovascular Outcomes in the AFFIRM Trial (Atrial Fibrillation Follow-Up Investigation of Rhythm Management) J. Am. Coll. Cardiol. 2011;58:1975–1985. doi: 10.1016/j.jacc.2011.07.036. - DOI - PMC - PubMed
-
- Allen LaPointe N.M., Dai D., Thomas L., Piccini J.P., Peterson E.D., Al-Khatib S.M. Comparisons of Hospitalization Rates Among Younger Atrial Fibrillation Patients Receiving Different Antiarrhythmic Drugs. Circ. Cardiovasc. Qual. Outcomes. 2015;8:292–300. doi: 10.1161/CIRCOUTCOMES.114.001499. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous