Programmed Death Ligand-1 (PD-L1) Is an Independent Negative Prognosticator in Western-World Gallbladder Cancer
- PMID: 33918309
- PMCID: PMC8038183
- DOI: 10.3390/cancers13071682
Programmed Death Ligand-1 (PD-L1) Is an Independent Negative Prognosticator in Western-World Gallbladder Cancer
Abstract
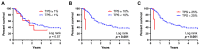
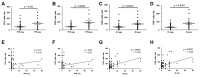
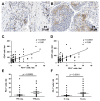
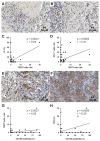
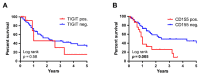
Inhibition of the programmed cell death protein-1/ligand-1 (PD-1/PD-L1) axis has opened a new era in the treatment of solid cancers. However, there is no data on the expression and relevance of PD-L1 in Western gallbladder cancer (GBC). We assessed PD-L1 immunohistochemically in 131 GBC patients as Tumor Proportion Score (TPS), Immune Cell Score (IC) and Combined Positivity Score (CPS). Tumor cells expressed PD-L1 in a subset of 14.7% GBC patients at a TPS cut-off of 1%. Higher PD-L1 levels above 10% and 25% TPS were reached in 4.7% and 3.1% of GBC cases, respectively. At a 10% cut-off, TPS was associated with distinct histomorphological subtypes and correlated with poor tumor differentiation. Survival analysis revealed a TPS above 10% to be a highly significant and independent negative prognosticator in GBC. PD-L1 expression was associated with increased CD4+, CD8+ and PD-1+ immune cell densities. In 14.8% of the cases, scattered immune cells expressed T-cell immunoreceptor with Ig and ITIM domains (TIGIT), which was correlated to tumoral expression of its ligand CD155. We here show that a high PD-L1 expression confers a negative prognostic value in Western-world GBC and highlight the TIGIT/CD155 immune checkpoint as a potential new target for GBC immunotherapy.
Keywords: PD-L1; biomarkers; gallbladder cancer; gastrointestinal neoplasms; immune evasion; liver neoplasms; programmed cell death ligand-1; tumor.
Conflict of interest statement
P.S. received funding for grants, boards, and presentations from Novartis. The other authors declare no competing interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

