Evaluation of the Variability of the ORF34, ORF68, and MLST Genes in EHV-1 from South Korea
- PMID: 33918404
- PMCID: PMC8066002
- DOI: 10.3390/pathogens10040425
Evaluation of the Variability of the ORF34, ORF68, and MLST Genes in EHV-1 from South Korea
Abstract
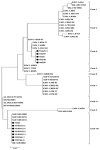
Equine herpesvirus-1 (EHV-1) is an important pathogen in horses. It affects horses worldwide and causes substantial economic losses. In this study, for the first time, we characterized EHV-1 isolates from South Korea at the molecular level. We then aimed to determine the genetic divergences of these isolates by comparing them to sequences in databases. In total, 338 horse samples were collected, and 12 EHV-1 were isolated. We performed ORF30, ORF33, ORF68, and ORF34 genetic analysis and carried out multi-locus sequence typing (MLST) of 12 isolated EHV-1. All isolated viruses were confirmed as non-neuropathogenic type, showing N752 of ORF30 and highly conserved ORF33 (99.7-100%). Isolates were unclassified using ORF68 analysis because of a 118 bp deletion in nucleotide sequence 701-818. Seven EHV-1 isolates (16Q4, 19R166-1, 19R166-6, 19/10/15-2, 19/10/15-4, 19/10/18-2, 19/10/22-1) belonged to group 1, clade 10, based on ORF34 and MLST analysis. The remaining 5 EHV-1 isolates (15Q25-1, 15D59, 16Q5, 16Q40, 18D99) belonged to group 7, clade 6, based on ORF34 and MLST analysis.
Keywords: EHV-1; MLST; ORF30; ORF33; ORF34; ORF68; phylogeny.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Equid alphaherpesvirus 1 from Italian Horses: Evaluation of the Variability of the ORF30, ORF33, ORF34 and ORF68 Genes.Viruses. 2019 Sep 13;11(9):851. doi: 10.3390/v11090851. Viruses. 2019. PMID: 31540321 Free PMC article.
-
Comparing the genetic diversity of ORF30 of Australian isolates of 3 equid alphaherpesviruses.Vet Microbiol. 2014 Feb 21;169(1-2):50-7. doi: 10.1016/j.vetmic.2013.12.007. Epub 2013 Dec 25. Vet Microbiol. 2014. PMID: 24418044
-
Molecular characterisation of equid alphaherpesvirus 1 strains isolated from aborted fetuses in Poland.Virol J. 2018 Dec 3;15(1):186. doi: 10.1186/s12985-018-1093-5. Virol J. 2018. PMID: 30509297 Free PMC article.
-
Molecular characterization of Brazilian equid herpesvirus type 1 strains based on neuropathogenicity markers.Braz J Microbiol. 2015 Jun 1;46(2):565-70. doi: 10.1590/S1517-838246220140096. eCollection 2015 Jun. Braz J Microbiol. 2015. PMID: 26273275 Free PMC article.
-
Molecular characterisation of the ORF68 region of equine herpesvirus-1 strains isolated from aborted fetuses in Hungary between 1977 and 2008.Acta Vet Hung. 2012 Mar;60(1):175-87. doi: 10.1556/AVet.2012.015. Acta Vet Hung. 2012. PMID: 22366142
Cited by
-
Molecular and Serological Investigation of Equine Herpesvirus Type 1 (EHV-1) and Type 4 (EHV-4) in Horses In Ibagué, Tolima.Vet Med Int. 2025 Jan 29;2025:1661949. doi: 10.1155/vmi/1661949. eCollection 2025. Vet Med Int. 2025. PMID: 39949613 Free PMC article.
-
Molecular characteristics and pathogenicity of an equid alphaherpesvirus 1 strain isolated in China.Virus Genes. 2022 Aug;58(4):284-293. doi: 10.1007/s11262-022-01910-y. Epub 2022 May 14. Virus Genes. 2022. PMID: 35567668
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

