Development of a Multimatrix UHPLC-MS/MS Method for the Determination of Paracetamol and Its Metabolites in Animal Tissues
- PMID: 33918518
- PMCID: PMC8038326
- DOI: 10.3390/molecules26072046
Development of a Multimatrix UHPLC-MS/MS Method for the Determination of Paracetamol and Its Metabolites in Animal Tissues
Abstract
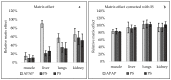
Paracetamol/acetaminophen (APAP) is one of the most popular pharmacologically active substances used as an analgesic and antipyretic agent. The metabolism of this drug occurs in the liver and leads to the formation of two main metabolites-glucuronic acid and sulfate derivate. Despite the wide use of paracetamol in veterinary medicine, a handful of analytical methods were published for the determination of paracetamol residues in animal tissues. In this paper, a multimatrix method has been developed for the determination of paracetamol and two metabolites-paracetamol sulfate (PS) and p-Acetamidophenyl β-D-glucuronide (PG). A validation procedure was conducted to verify method reliability and fit purpose as a tool for analyzing acetaminophen and metabolites in muscle, liver, lung, and kidney samples from different species of animals. Established validation parameters were in agreement with acceptable criteria laid by the European legislation. The initial significant matrix effect was successfully reduced by implementing an internal standard-4-Acetamidophenyl β-D-glucuronide-d3 (PG-d3, IS). The usefulness of the developed method was verified by analyzing samples from an experiment in which paracetamol was administrated to geese.
Keywords: UHPLC-MS/MS; heart; kidneys; liver; lungs; metabolites; muscle; paracetamol; residues.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Malaise O., Bruyere O., Reginster J.Y. Intravenous paracetamol: A review of efficacy and safety in therapeutic use. Future Neurol. 2007;2:673–688. doi: 10.2217/14796708.2.6.673. - DOI
-
- Jozwiak-Bebenista M., Nowak J.Z. Paracetamol: Mechanism of action, applications and safety concern. Acta Pol. Pharm. Drug Res. 2014;71:11–23. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

