IL-1β Antibody Protects Brain from Neuropathology of Hypoperfusion
- PMID: 33918659
- PMCID: PMC8069995
- DOI: 10.3390/cells10040855
IL-1β Antibody Protects Brain from Neuropathology of Hypoperfusion
Abstract
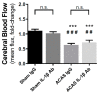
Chronic brain hypoperfusion is the primary cause of vascular dementia and has been implicated in the development of white matter disease and lacunar infarcts. Cerebral hypoperfusion leads to a chronic state of brain inflammation with immune cell activation and production of pro-inflammatory cytokines, including IL-1β. In the present study, we induced chronic, progressive brain hypoperfusion in mice using ameroid constrictor, arterial stenosis (ACAS) surgery and tested the efficacy of an IL-1β antibody on the resulting brain damage. We observed that ACAS surgery causes a reduction in cerebral blood flow (CBF) of about 30% and grey and white matter damage in and around the hippocampus. The IL-1β antibody treatment did not significantly affect CBF but largely eliminated grey matter damage and reduced white matter damage caused by ACAS surgery. Over the course of hypoperfusion/injury, grip strength, coordination, and memory-related behavior were not significantly affected by ACAS surgery or antibody treatment. We conclude that antibody neutralization of IL-1β is protective from the brain damage caused by chronic, progressive brain hypoperfusion.
Keywords: IL-1β; IL-1β antibody; canakinumab; cerebral hypoperfusion; white matter damage and grey matter damage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Kynast J., Lampe L., Luck T., Frisch S., Arelin K., Hoffmann K.-T., Loeffler M., Riedel-Heller S.G., Villringer A., Schroeter M.L. White matter hyperintensities associated with small vessel disease impair social cognition beside attention and memory. Br. J. Pharmacol. 2018;38:996–1009. doi: 10.1177/0271678X17719380. - DOI - PMC - PubMed
-
- Borroni B., Perani D., Broli M., Colciaghi F., Garibotto V., Paghera B., Agosti C., Giubbini R., Di Luca M., Padovani A. Pre–clinical diagnosis of Alzheimer disease combining platelet amyloid precursor protein ratio and rCBF spect analysis. J. Neurol. 2005;252:1359–1362. doi: 10.1007/s00415-005-0867-z. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

