JNK Pathway in CNS Pathologies
- PMID: 33918666
- PMCID: PMC8070500
- DOI: 10.3390/ijms22083883
JNK Pathway in CNS Pathologies
Abstract
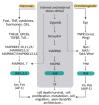
The c-Jun N-terminal kinase (JNK) signalling pathway is a conserved response to a wide range of internal and external cellular stress signals. Beside the stress response, the JNK pathway is involved in a series of vital regulatory mechanisms during development and adulthood that are critical to maintain tissue homeostasis. These mechanisms include the regulation of apoptosis, growth, proliferation, differentiation, migration and invasion. The JNK pathway has a diverse functionality and cell-tissue specificity, and has emerged as a key player in regeneration, tumorigenesis and other pathologies. The JNK pathway is highly active in the central nervous system (CNS), and plays a central role when cells need to cope with pathophysiological insults during development and adulthood. Here, we review the implications of the JNK pathway in pathologies of the CNS. More specifically, we discuss some newly identified examples and mechanisms of JNK-driven tumor progression in glioblastoma, regeneration/repair after an injury, neurodegeneration and neuronal cell death. All these new discoveries support the central role of JNK in CNS pathologies and reinforce the idea of JNK as potential target to reduce their detrimental effects.
Keywords: CNS injuries; MAP kinase; cell signalling; glioblastoma; neurodegenerative disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Hamdi M., Kool J., Cornelissen-Steijger P., Carlotti F., Popeijus H.E., Van Der Burgt C., Janssen J.M., Yasui A., Hoeben R.C., Terleth C., et al. DNA damage in transcribed genes induces apoptosis via the JNK pathway and the JNK-phosphatase MKP-1. Oncogene. 2005;24:7135–7144. doi: 10.1038/sj.onc.1208875. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

