A Systematic Review of Glioblastoma-Targeted Therapies in Phases II, III, IV Clinical Trials
- PMID: 33918704
- PMCID: PMC8069979
- DOI: 10.3390/cancers13081795
A Systematic Review of Glioblastoma-Targeted Therapies in Phases II, III, IV Clinical Trials
Abstract
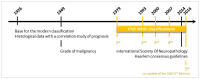
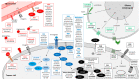
Glioblastoma (GBM), the most frequent and aggressive glial tumor, is currently treated as first line by the Stupp protocol, which combines, after surgery, radiotherapy and chemotherapy. For recurrent GBM, in absence of standard treatment or available clinical trials, various protocols including cytotoxic drugs and/or bevacizumab are currently applied. Despite these heavy treatments, the mean overall survival of patients is under 18 months. Many clinical studies are underway. Based on clinicaltrials.org and conducted up to 1 April 2020, this review lists, not only main, but all targeted therapies in phases II-IV of 257 clinical trials on adults with newly diagnosed or recurrent GBMs for the last twenty years. It does not involve targeted immunotherapies and therapies targeting tumor cell metabolism, that are well documented in other reviews. Without surprise, the most frequently reported drugs are those targeting (i) EGFR (40 clinical trials), and more generally tyrosine kinase receptors (85 clinical trials) and (ii) VEGF/VEGFR (75 clinical trials of which 53 involving bevacizumab). But many other targets and drugs are of interest. They are all listed and thoroughly described, on an one-on-one basis, in four sections related to targeting (i) GBM stem cells and stem cell pathways, (ii) the growth autonomy and migration, (iii) the cell cycle and the escape to cell death, (iv) and angiogenesis.
Keywords: biomarkers; clinical trials; glioblastoma; targeted therapies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Stupp R., Hegi M.E., Mason W.P., van den Bent M.J., Taphoorn M.J.B., Janzer R.C., Ludwin S.K., Allgeier A., Fisher B., Belanger K., et al. Effects of Radiotherapy with Concomitant and Adjuvant Temozolomide versus Radiotherapy Alone on Survival in Glioblastoma in a Randomised Phase III Stud y: 5-Year Analysis of the EORTC-NCIC Trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

