Hypoxic Conditions Promote the Angiogenic Potential of Human Induced Pluripotent Stem Cell-Derived Extracellular Vesicles
- PMID: 33918735
- PMCID: PMC8070165
- DOI: 10.3390/ijms22083890
Hypoxic Conditions Promote the Angiogenic Potential of Human Induced Pluripotent Stem Cell-Derived Extracellular Vesicles
Abstract
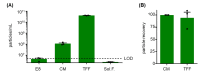
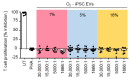
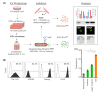
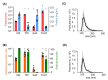
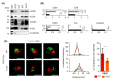
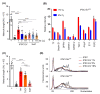
Stem cells secrete paracrine factors including extracellular vesicles (EVs) which can mediate cellular communication and support the regeneration of injured tissues. Reduced oxygen (hypoxia) as a key regulator in development and regeneration may influence cellular communication via EVs. We asked whether hypoxic conditioning during human induced pluripotent stem cell (iPSC) culture effects their EV quantity, quality or EV-based angiogenic potential. We produced iPSC-EVs from large-scale culture-conditioned media at 1%, 5% and 18% air oxygen using tangential flow filtration (TFF), with or without subsequent concentration by ultracentrifugation (TUCF). EVs were quantified by tunable resistive pulse sensing (TRPS), characterized according to MISEV2018 guidelines, and analyzed for angiogenic potential. We observed superior EV recovery by TFF compared to TUCF. We confirmed hypoxia efficacy by HIF-1α stabilization and pimonidazole hypoxyprobe. EV quantity did not differ significantly at different oxygen conditions. Significantly elevated angiogenic potential was observed for iPSC-EVs derived from 1% oxygen culture by TFF or TUCF as compared to EVs obtained at higher oxygen or the corresponding EV-depleted soluble factor fractions. Data thus demonstrate that cell-culture oxygen conditions and mode of EV preparation affect iPSC-EV function. We conclude that selecting appropriate protocols will further improve production of particularly potent iPSC-EV-based therapeutics.
Keywords: angiogenesis; extracellular vesicles (EV); hypoxia; hypoxia-inducible transcription factor (HIF); induced pluripotent stem cells (iPSC); regenerative medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Reinisch A., Hofmann N.A.N.A., Obenauf A.C.A.C., Kashofer K., Rohde E., Schallmoser K., Flicker K., Lanzer G., Linkesch W., Speicher M.R.M.R., et al. Humanized large-scale expanded endothelial colony-forming cells function in vitro and in vivo. Blood. 2009;113:6716–6725. doi: 10.1182/blood-2008-09-181362. - DOI - PMC - PubMed
-
- Prasain N., Lee M.R., Vemula S., Meador J.L., Yoshimoto M., Ferkowicz M.J., Fett A., Gupta M., Rapp B.M., Saadatzadeh M.R., et al. Differentiation of human pluripotent stem cells to cells similar to cord-blood endothelial colony-forming cells. Nat. Biotechnol. 2014;32:1151–1157. doi: 10.1038/nbt.3048. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

