Silver Nanoparticles as Carriers of Anticancer Drugs for Efficient Target Treatment of Cancer Cells
- PMID: 33918740
- PMCID: PMC8069134
- DOI: 10.3390/nano11040964
Silver Nanoparticles as Carriers of Anticancer Drugs for Efficient Target Treatment of Cancer Cells
Abstract
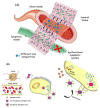
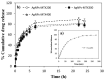
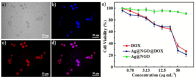
Since the last decade, nanotechnology has evolved rapidly and has been applied in several areas, such as medicine, pharmaceutical, microelectronics, aerospace, food industries, among others. The use of nanoparticles as drug carriers has been explored and presents several advantages, such as controlled and targeted release of loaded or coupled drugs, and the improvement of the drug's bioavailability, in addition to others. However, they also have some limitations, related to their in vivo toxicity, which affects all organs including the healthy ones, and overall improvement in the disease treatment, which can be unnoticeable or minimal. Silver nanoparticles have been increasingly investigated due to their peculiar physical, chemical, and optical properties, which allows them to cover several applications, namely in the transport of drugs to a specific target in the body. Given the limitations of conventional cancer chemotherapy, which include low bioavailability and the consequent use of high doses that cause adverse effects, strategies that overcome these difficulties are extremely important. This review embraces an overview and presentation about silver nanoparticles used as anticancer drug carrier systems and focuses a discussion on the state of the art of silver nanoparticles exploited for transport of anticancer drugs and their influence on antitumor effects.
Keywords: anticancer drugs; antitumor effects; nanocarriers; silver nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Gali-Muhtasib H., Chouaib R. Nanoparticle Drug Delivery Systems for Cancer Treatment. 1st ed. Jenny Stanford Publishing; Boca Raton, FL, USA: 2020. Nanoparticles in cancer treatment: Types and preparation methods.
-
- Ealia A.M., Saravanakumar M.P. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017;263:032019. doi: 10.1088/1757-899X/263/3/032019. - DOI
-
- Gali-Muhtasib H., Chouaib R. Nanoparticle Drug Delivery Systems for Cancer Treatment. 1st ed. Jenny Stanford Publishing; Boca Raton, FL, USA: 2020. Anti-tumor activity of verbascoside-loaded noble metal nanoparticles.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

