Electroosmotic Flow of Viscoelastic Fluid through a Constriction Microchannel
- PMID: 33918910
- PMCID: PMC8069235
- DOI: 10.3390/mi12040417
Electroosmotic Flow of Viscoelastic Fluid through a Constriction Microchannel
Abstract
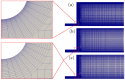
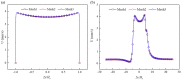
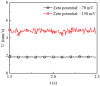
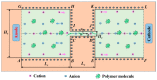
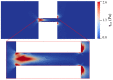
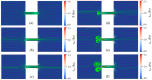
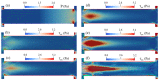
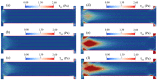
Electroosmotic flow (EOF) has been widely used in various biochemical microfluidic applications, many of which use viscoelastic non-Newtonian fluid. This study numerically investigates the EOF of viscoelastic fluid through a 10:1 constriction microfluidic channel connecting two reservoirs on either side. The flow is modelled by the Oldroyd-B (OB) model coupled with the Poisson-Boltzmann model. EOF of polyacrylamide (PAA) solution is studied as a function of the PAA concentration and the applied electric field. In contrast to steady EOF of Newtonian fluid, the EOF of PAA solution becomes unstable when the applied electric field (PAA concentration) exceeds a critical value for a fixed PAA concentration (electric field), and vortices form at the upstream of the constriction. EOF velocity of viscoelastic fluid becomes spatially and temporally dependent, and the velocity at the exit of the constriction microchannel is much higher than that at its entrance, which is in qualitative agreement with experimental observation from the literature. Under the same apparent viscosity, the time-averaged velocity of the viscoelastic fluid is lower than that of the Newtonian fluid.
Keywords: Oldroyd-B model; elastic instability; electroosmosis; microfluidics; non-Newtonian fluid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






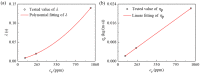

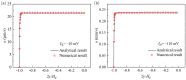

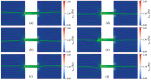
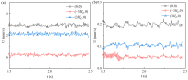




Similar articles
-
Electro-Elastic Instability and Turbulence in Electro-osmotic Flows of Viscoelastic Fluids: Current Status and Future Directions.Micromachines (Basel). 2025 Feb 4;16(2):187. doi: 10.3390/mi16020187. Micromachines (Basel). 2025. PMID: 40047668 Free PMC article. Review.
-
Numerical Study of the Time-Periodic Electroosmotic Flow of Viscoelastic Fluid through a Short Constriction Microchannel.Micromachines (Basel). 2023 Nov 8;14(11):2077. doi: 10.3390/mi14112077. Micromachines (Basel). 2023. PMID: 38004934 Free PMC article.
-
Electroosmotic flow of non-Newtonian fluids in a constriction microchannel.Electrophoresis. 2019 May;40(10):1387-1394. doi: 10.1002/elps.201800315. Epub 2018 Nov 6. Electrophoresis. 2019. PMID: 30346029
-
Elastic instabilities in the electroosmotic flow of non-Newtonian fluids through T-shaped microchannels.Electrophoresis. 2020 Apr;41(7-8):588-597. doi: 10.1002/elps.201900331. Epub 2019 Dec 11. Electrophoresis. 2020. PMID: 31786811
-
The Electroosmotic Flow (EOF).Methods Mol Biol. 2010;583:121-34. doi: 10.1007/978-1-60327-106-6_5. Methods Mol Biol. 2010. PMID: 19763462 Review.
Cited by
-
Microfluidic Mixing: A Physics-Oriented Review.Micromachines (Basel). 2023 Sep 25;14(10):1827. doi: 10.3390/mi14101827. Micromachines (Basel). 2023. PMID: 37893264 Free PMC article. Review.
-
A simple yet efficient approach for electrokinetic mixing of viscoelastic fluids in a straight microchannel.Sci Rep. 2022 Feb 14;12(1):2395. doi: 10.1038/s41598-022-06202-x. Sci Rep. 2022. PMID: 35165299 Free PMC article.
-
Review on Blood Flow Dynamics in Lab-on-a-Chip Systems: An Engineering Perspective.Chem Bio Eng. 2024 Jan 4;1(1):26-43. doi: 10.1021/cbe.3c00014. eCollection 2024 Feb 22. Chem Bio Eng. 2024. PMID: 39973974 Free PMC article. Review.
-
Electro-Elastic Instability and Turbulence in Electro-osmotic Flows of Viscoelastic Fluids: Current Status and Future Directions.Micromachines (Basel). 2025 Feb 4;16(2):187. doi: 10.3390/mi16020187. Micromachines (Basel). 2025. PMID: 40047668 Free PMC article. Review.
-
Editorial for the Special Issue on Micromachines for Non-Newtonian Microfluidics.Micromachines (Basel). 2022 Jun 8;13(6):906. doi: 10.3390/mi13060906. Micromachines (Basel). 2022. PMID: 35744520 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

