Prostaglandin F2 Alpha Triggers the Disruption of Cell Adhesion with Cytokeratin and Vimentin in Bovine Luteal Theca Cells
- PMID: 33918916
- PMCID: PMC8069824
- DOI: 10.3390/ani11041073
Prostaglandin F2 Alpha Triggers the Disruption of Cell Adhesion with Cytokeratin and Vimentin in Bovine Luteal Theca Cells
Abstract
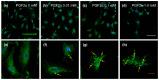
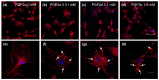
Intermediate filaments (IFs) maintain cell-cell adhesions and are involved in diverse cellular processes such as cytokinesis, cell migration and the maintenance of cell structure. In this study, we investigated the influence of prostaglandin F2 alpha (PGF2α) on cytokeratin and vimentin IFs, Rho-associated protein kinase (ROCK), and cell-cell adhesion in bovine luteal theca cells (LTCs). The luteal cells were isolated from bovine corpus luteum (CL), and the LTCs were treated with 0, 0.01, 0.1 and 1.0 mM PGF2α. Cytokeratin, vimentin and desmoplakin proteins were disrupted and the ROCK protein was significantly increased in PGF2α-treated LTCs. In addition, cell-cell adhesion was significantly (p < 0.05) decreased in the PGF2α-induced LTCs compared to control group (0 mM PGF2α). In conclusion, PGF2α affected the adhesion of cell to cell via disruption of desmoplakin, cytokeratin and vimentin, additionally increasing ROCK in bovine LTCs. These results may provide a better understanding of the mechanism of bovine CL regression.
Keywords: cell adhesion; corpus luteum; luteolysis; ovary; prostaglandin F2 alpha.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Deciphering the functional role of EGR1 in Prostaglandin F2 alpha induced luteal regression applying CRISPR in corpus luteum of buffalo.Biol Res. 2021 Mar 12;54(1):9. doi: 10.1186/s40659-021-00333-7. Biol Res. 2021. PMID: 33712084 Free PMC article.
-
Expression of prostaglandin F2α (PGF2α) receptor and its isoforms in the bovine corpus luteum during the estrous cycle and PGF2α-induced luteolysis.Domest Anim Endocrinol. 2012 Oct;43(3):227-38. doi: 10.1016/j.domaniend.2012.03.003. Epub 2012 Apr 12. Domest Anim Endocrinol. 2012. PMID: 22560179
-
The effect of basic fibroblast growth factor 2 on the bovine corpus luteum depends on the stage of the estrous cycle and modulates prostaglandin F2α action.Animal. 2021 Jan;15(1):100048. doi: 10.1016/j.animal.2020.100048. Epub 2020 Dec 10. Animal. 2021. PMID: 33516003
-
Inter-relationships between endothelin and prostaglandin F2alpha in corpus luteum function.Rev Reprod. 2000 Jan;5(1):1-5. doi: 10.1530/ror.0.0050001. Rev Reprod. 2000. PMID: 10711729 Review.
-
Inter- and intra-cellular mechanisms of prostaglandin F2alpha action during corpus luteum regression in cattle.Soc Reprod Fertil Suppl. 2010;67:305-24. doi: 10.7313/upo9781907284991.025. Soc Reprod Fertil Suppl. 2010. PMID: 21755681 Review.
References
-
- Berisha B., Bridger P., Toth A., Kliem H., Meyer H., Schams D., Pfarrer C. Expression and localization of gap junctional connexins 26 and 43 in bovine periovulatory follicles and in corpus luteum during different functional stages of oestrous cycle and pregnancy. Reprod. Domest. Anim. 2009;44:295–302. doi: 10.1111/j.1439-0531.2008.01068.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

