Nanomaterials in Wound Healing and Infection Control
- PMID: 33919072
- PMCID: PMC8143158
- DOI: 10.3390/antibiotics10050473
Nanomaterials in Wound Healing and Infection Control
Abstract
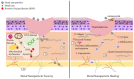
Wounds continue to be a serious medical concern due to their increasing incidence from injuries, surgery, burns and chronic diseases such as diabetes. Delays in the healing process are influenced by infectious microbes, especially when they are in the biofilm form, which leads to a persistent infection. Biofilms are well known for their increased antibiotic resistance. Therefore, the development of novel wound dressing drug formulations and materials with combined antibacterial, antibiofilm and wound healing properties are required. Nanomaterials (NM) have unique properties due to their size and very large surface area that leads to a wide range of applications. Several NMs have antimicrobial activity combined with wound regeneration features thus give them promising applicability to a variety of wound types. The idea of NM-based antibiotics has been around for a decade at least and there are many recent reviews of the use of nanomaterials as antimicrobials. However, far less attention has been given to exploring if these NMs actually improve wound healing outcomes. In this review, we present an overview of different types of nanomaterials explored specifically for wound healing properties combined with infection control.
Keywords: antimicrobial activity; antimicrobial nanomaterials; infection control; infectious microbes; nanoparticles; wound healing; wound management.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Jahromi M.A.M., Zangabad P.S., Basri S.M.M., Zangabad K.S., Ghamarypour A., Aref A.R., Karimi M., Hamblin M.R. Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv. Drug Deliv. Rev. 2018;123:33–64. doi: 10.1016/j.addr.2017.08.001. - DOI - PMC - PubMed
-
- Deepachitra R., Lakshmi R.P., Sivaranjani K., Chandra J.H., Sastry T.P. Nanoparticles embedded biomaterials in wound treatment: A review. J. Chem. Pharm. Sci. 2015;8:324–329.
-
- Akers K.S., Wenke J.C., Murray C.K. Biofilms and Wound Infection Research in the US Military. In: Williams D., editor. Targeting Biofilms in Translational Research, Device Development, and Industrial Sectors. Springer; Berlin/Heidelberg, Germany: 2019. pp. 55–69.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

