Hypoxia-Induced FAM13A Regulates the Proliferation and Metastasis of Non-Small Cell Lung Cancer Cells
- PMID: 33919074
- PMCID: PMC8122400
- DOI: 10.3390/ijms22094302
Hypoxia-Induced FAM13A Regulates the Proliferation and Metastasis of Non-Small Cell Lung Cancer Cells
Abstract
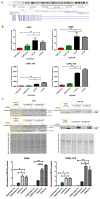
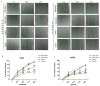
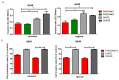
Hypoxia in non-small cell lung cancer (NSCLC) affects cancer progression, metastasis and metabolism. We previously showed that FAM13A was induced by hypoxia in NSCLC but the biological function of this gene has not been fully elucidated. This study aimed to investigate the role of hypoxia-induced FAM13A in NSCLC progression and metastasis. Lentiviral shRNAs were used for FAM13A gene silencing in NSCLC cell lines (A549, CORL-105). MTS assay, cell tracking VPD540 dye, wound healing assay, invasion assay, BrdU assay and APC Annexin V staining assays were performed to examine cell proliferation ability, migration, invasion and apoptosis rate in NSCLC cells. The results of VPD540 dye and MTS assays showed a significant reduction in cell proliferation after FAM13A knockdown in A549 cells cultured under normal and hypoxia (1% O2) conditions (p < 0.05), while the effect of FAM13A downregulation on CORL-105 cells was observed after 96 h exposition to hypoxia. Moreover, FAM13A inhibition induced S phase cell cycle arrest in A549 cells under hypoxia conditions. Silencing of FAM13A significantly suppressed migration of A549 and CORL-105 cells in both oxygen conditions, especially after 72 and 96 h (p < 0.001 in normoxia, p < 0.01 after hypoxia). It was showed that FAM13A reduction resulted in disruption of the F-actin cytoskeleton altering A549 cell migration. Cell invasion rates were significantly decreased in A549 FAM13A depleted cells compared to controls (p < 0.05), mostly under hypoxia. FAM13A silencing had no effect on apoptosis induction in NSCLC cells. In the present study, we found that FAM13A silencing has a negative effect on proliferation, migration and invasion activity in NSCLC cells in normal and hypoxic conditions. Our data demonstrated that FAM13A depleted post-hypoxic cells have a decreased cell proliferation ability and metastatic potential, which indicates FAM13A as a potential therapeutic target in lung cancer.
Keywords: FAM13A gene; cell migration; cell proliferation; hypoxia; invasion; non-small cell lung cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Minakata K., Takahashi F., Nara T., Hashimoto M., Tajima K., Murakami A., Nurwidya F., Yae S., Koizumi F., Moriyama H., et al. Hypoxia induces gefitinib resistance in non-small-cell lung cancer with both mutant and wild-type epidermal growth factor receptors. Cancer Sci. 2012;103:1946–1954. doi: 10.1111/j.1349-7006.2012.02408.x. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

