Coxsackievirus B3-Its Potential as an Oncolytic Virus
- PMID: 33919076
- PMCID: PMC8143167
- DOI: 10.3390/v13050718
Coxsackievirus B3-Its Potential as an Oncolytic Virus
Abstract
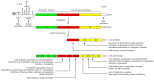
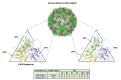
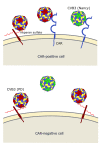
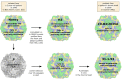
Oncolytic virotherapy represents one of the most advanced strategies to treat otherwise untreatable types of cancer. Despite encouraging developments in recent years, the limited fraction of patients responding to therapy has demonstrated the need to search for new suitable viruses. Coxsackievirus B3 (CVB3) is a promising novel candidate with particularly valuable features. Its entry receptor, the coxsackievirus and adenovirus receptor (CAR), and heparan sulfate, which is used for cellular entry by some CVB3 variants, are highly expressed on various cancer types. Consequently, CVB3 has broad anti-tumor activity, as shown in various xenograft and syngeneic mouse tumor models. In addition to direct tumor cell killing the virus induces a strong immune response against the tumor, which contributes to a substantial increase in the efficiency of the treatment. The toxicity of oncolytic CVB3 in healthy tissues is variable and depends on the virus strain. It can be abrogated by genetic engineering the virus with target sites of microRNAs. In this review, we present an overview of the current status of the development of CVB3 as an oncolytic virus and outline which steps still need to be accomplished to develop CVB3 as a therapeutic agent for clinical use in cancer treatment.
Keywords: Coxsackievirus B3; cancer; miR; microRNA; oncolytic virus; virus adaptation.
Conflict of interest statement
H.F. and A.H. have patented the CVB3 variant PD for use in cancer therapy and have a patent pending for treatment of cancer using miR-TS in oncolytic CVB3. All other authors declare no conflicts of interest.
Figures


Similar articles
-
Oncolytic activity of a coxsackievirus B3 strain in patient-derived cervical squamous cell carcinoma organoids and synergistic effect with paclitaxel.Virol J. 2024 Oct 5;21(1):245. doi: 10.1186/s12985-024-02502-y. Virol J. 2024. PMID: 39369233 Free PMC article.
-
Heparan Sulfate Binding Coxsackievirus B3 Strain PD: A Novel Avirulent Oncolytic Agent Against Human Colorectal Carcinoma.Hum Gene Ther. 2018 Nov;29(11):1301-1314. doi: 10.1089/hum.2018.036. Epub 2018 Jun 20. Hum Gene Ther. 2018. PMID: 29739251
-
Development of New Oncolytic Virotherapy Targeting Breast Cancer Using Coxsackievirus B3.Anticancer Res. 2021 Jan;41(1):81-89. doi: 10.21873/anticanres.14753. Anticancer Res. 2021. PMID: 33419801
-
[Oncolytic coxsackievirus therapy as an immunostimulator].Rinsho Ketsueki. 2017;58(8):977-982. doi: 10.11406/rinketsu.58.977. Rinsho Ketsueki. 2017. PMID: 28883283 Review. Japanese.
-
Adenovirus Receptor Expression in Cancer and Its Multifaceted Role in Oncolytic Adenovirus Therapy.Int J Mol Sci. 2020 Sep 17;21(18):6828. doi: 10.3390/ijms21186828. Int J Mol Sci. 2020. PMID: 32957644 Free PMC article. Review.
Cited by
-
Oncolytic Viruses and Immune Checkpoint Inhibitors: The "Hot" New Power Couple.Cancers (Basel). 2023 Aug 19;15(16):4178. doi: 10.3390/cancers15164178. Cancers (Basel). 2023. PMID: 37627206 Free PMC article. Review.
-
Combination of FOLFOXIRI Drugs with Oncolytic Coxsackie B3 Virus PD-H Synergistically Induces Oncolysis in the Refractory Colorectal Cancer Cell Line Colo320.Int J Mol Sci. 2024 May 22;25(11):5618. doi: 10.3390/ijms25115618. Int J Mol Sci. 2024. PMID: 38891807 Free PMC article.
-
Oncolytic activity of a coxsackievirus B3 strain in patient-derived cervical squamous cell carcinoma organoids and synergistic effect with paclitaxel.Virol J. 2024 Oct 5;21(1):245. doi: 10.1186/s12985-024-02502-y. Virol J. 2024. PMID: 39369233 Free PMC article.
-
Non-small cell lung cancers (NSCLCs) oncolysis using coxsackievirus B5 and synergistic DNA-damage response inhibitors.Signal Transduct Target Ther. 2023 Sep 25;8(1):366. doi: 10.1038/s41392-023-01603-4. Signal Transduct Target Ther. 2023. PMID: 37743418 Free PMC article.
-
Breaking the barriers in cancer care: The next generation of herpes simplex virus-based oncolytic immunotherapies for cancer treatment.Mol Ther Oncolytics. 2023 Sep 19;31:100729. doi: 10.1016/j.omto.2023.100729. eCollection 2023 Dec 19. Mol Ther Oncolytics. 2023. PMID: 37841530 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

